A review of parathyroid autofluorescence imaging in detection and preservation of parathyroid glands during thyroid surgery
Introduction
Background
In recent years, the incidence of thyroid cancer has increased. Currently, it is the most frequent endocrine related malignancy with up to 14.3 cases per 100,000 individuals (1). Surgery is the cornerstone treatment, and although mortality is almost null, patients are still prone to complications (2,3). After thyroidectomy, transient hypocalcemia can occur in up to 51% of the patients which can be persistent in 4% of the cases (4). As a result of these increased postoperative rates of hypocalcemia, surgeons performing thyroidectomy had focused on ways to improve their results.
Rationale and knowledge gap
Although it has been shown that high-volume surgeons have better overall outcomes, the majority of thyroidectomies are still being performed by low-volume surgeons, and even in specialized centers the rate of incidental parathyroidectomy during thyroidectomy is about 16% (5,6). It has been postulated that the difficulty in localizing both normal and abnormal parathyroid glands (PGs) intraoperatively is due to its small size, variable location and inconspicuous color (7). In addition, their localization relies on visual inspection which is mostly dependent on the surgeon’s experience. In an effort to improve the identification of parathyroid tissue, the field of near-infrared autofluorescence (NIRAF) imaging was developed. Surgeons employing new technologies are required to be well versed in the concepts behind it. In addition, it is important to be aware of the timeframe which led to the technological developments.
Objective
As a recent manuscript has already perform a comprehensive systematic review and qualitative analysis of optical technologies during parathyroidectomy (8). The purpose of this review is to describe the landmark studies in a timeline fashion which led to the development of parathyroid autofluorescence (AF) and its application in thyroidectomy.
Near-infrared (NIR) light spectroscopy
Visible light refers to electromagnetic radiation in the form of wavelengths that stimulate the sight and make things visible (9). Although the human eye can only detect wavelengths from 380 to 760 nm, other wavelengths occur beyond this spectrum called NIR. The use of NIR in medicine dates back to 1977 when it was first used to probe the oxygenation state of living tissue, and since that time multiple other applications have been described (10). The first application of NIR in parathyroid tissue was done by Das et al. who used spectroscopy to differentiate between parathyroid adenomas from hyperplasia (11).
In this first study, an 830 nm diode laser was used as the excitation source for 698 PGs. This excitation laser light produced a frequency shift which was then measured by spectroscopy (11). The results demonstrated that the average point spectra between adenomas and hyperplasia was different enough to be able to distinguish between them with 93% sensitivity and specificity. Interestingly, this first report did not find the AF properties of the parathyroid tissue, which was likely due to the use of an 830 nm excitation laser (12).
Four years later, Paras et al. developed for the first time an optical method to be able to discriminate parathyroid tissue from other structures in the neck using NIR (12). In this pilot study, in vivo stimulation of PGs, thyroid, fat, muscle and trachea was done with a NIR 785 nm diode laser. Fluorescence spectra was then measured with a sterile fiber optic probe and compared between tissue samples. The results demonstrated that other tissues had no fluorescence when stimulated with NIR while the PGs had AF eleven times more intense than the thyroid. The primary fluorophore responsible for the AF was thought to be a calcium-sensing receptor widely found in PGs (13).
After demonstrating the AF properties of PGs, the same group from Vanderbilt University published their experience using this technique in 45 patients undergoing thyroidectomy and parathyroidectomy (13). In this study, the surgeon’s visual assessment was compared to the fluorescence detected by spectrometry and to histology which was used as the gold standard. Their results showed that in 3 cases out of 22, the surgeon incorrectly labeled the tissue while the fluorescence spectroscopy was able to identify it with 100% sensitivity and specificity. Results from this group also demonstrated that although NIRAF was able to correctly identify PGs, body mass index, disease state, vitamin D and calcium levels could contribute to signal variability between glands (14).
NIR light imaging
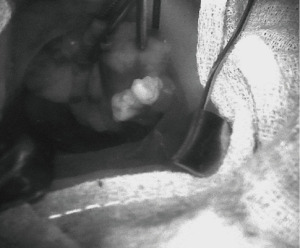
A follow-up study by McWade et al., which included 110 patients confirmed the accuracy of NIRAF to detect parathyroid tissue using spectroscopy. In addition to spectroscopy measurements, intraoperative fluorescence imaging was captured for 6 patients using a customized endoscope camera. These imaging results showed that PGs emit a strong fluorescence signal which was able to be seen on screen (Figure 1) (7). Nevertheless, the NIRAF image required the overlay of two separate images to identify the PGs. This limitation was addressed by Kim et al. by adding and infrared illuminator to the NIR imaging system which allowed real-time single image of the PGs as well as the background tissue (15).
Later on, Falco et al. utilized for the first time the Fluobeam (Fluoptics, Grenoble, France) in 28 patients undergoing parathyroidectomy and thyroidectomy (5). This tool emits NIR at 750 nm and then a special filter captures the fluorescence light in the range of 800 to 900 nm, allowing real time visualization of the surgical field. Although this camera classically uses indocyanine green as a fluorophore, the authors took advantage of the PGs AF properties for their study. The results demonstrated that PGs showed higher fluorescence compared to the background similar to that appreciated with spectroscopy.
Around the same time, De Leeuw et al. utilized the same commercial NIR imaging device as Falco et al. to visualize parathyroid tissue in vivo and ex vivo (16). They were able to identify 16 out of 17 PGs that were found on histopathology, interestingly the only one missed was found to be intrathyroidal. More importantly, their study found that parathyroid tissue remains AF ex vivo even after formalin fixation. In addition, in 5 cases NIRAF was able to identify the PGs before the surgeon. This main finding added up to the growing evidence of the feasibility and usefulness of NIRAF for intraoperative PGs identification. Nevertheless, in their study false positives were also noted arising from colloid nodules.
Following these institutional experiences using NIR imaging, a multicenter study was done to assess how this technology could facilitate the detection of PGs during both thyroidectomy and parathyroidectomy. The authors included 210 patients from three tertiary-care centers. The primary outcome measured was if the PGs were first detected by the surgeon’s naked eye or with NIRAF. The overall sensitivity observed for NIRAF was 98%, in addition, 46% of the cases the PGs were not seen by the naked eye due to coverage by soft tissue but were detectable with NIRAF without any further dissection (17). The authors concluded that NIRAF could be an adjunct for intraoperative PGs identification.
In a similar way, Ladurner et al. utilizing a different commercially available NIR system for indocyanine green (Karl Storz, Tittlingen, Germany) assessed the AF of PGs during parathyroidectomy and thyroidectomy (18,19). This system provided a wavelength of 690 to 770 nm and then a filter modified camera recorded the surgical field. Using this technique, the authors were able to reproduce the AF of the PGs when stimulated with NIR, however, there were some pitfalls as the camera was made to detect the fluorescence of indocyanine green and not that somewhat weaker AF of PGs (18). When the camera was used during thyroidectomy, the sensitivity to identify PGs was 90% and even in one case it recognized a gland during a central lymph node dissection which was preserved for auto transplantation (19).
Comparing NIRAF to other dyes
NIRAF is not the only technology which has been studied to help identify PGs during thyroidectomy. Actually, this technology is at its early stages compared to others such as methylene blue which have been used for over 40 years (8). Methylene blue, indocyanine green and 5-aminolevulinic acid have been used as adjuncts to PGs identification, nevertheless they are all prone to side effects from its administration (20,21). Contrastingly, NIRAF takes advantage of the intrinsic AF of the PGs avoiding the dangerous side effects of injecting other substances (22). Nevertheless, a disadvantage of NIRAF when compared to indocyanine green is the fact that AF using NIR persist ex vivo limiting the evaluation of PGs perfusion (Figure 2) (20).
Approval for intraoperative use
In 2018, the Food and Drug Administration approved two commercial systems for real-time parathyroid identification (22). The Fluobeam (Fluoptics, Grenoble, France), as previously described, relies on fluorescence imaging providing an image without quantification. On the other hand the PTeye (Medtronic, Minneapolis, MN, USA) relies on spectroscopy, providing a numerical value when the probe gets in contact with the tissue. This device was developed after the original studies by the Vanderbilt University group using spectroscopy. Currently there are no prospective trials comparing both technologies side to side making the selection of one over the other dependent on the surgeon’s preference.
In 2019, Thomas et al. reported their experience using PTeye (Medtronic, Minneapolis, MN, USA) which was able to be used with ambient operating room lights compared to prior spectroscopy devices (23). In this study, the Overlay Tissue Imaging System (OTIS) was evaluated as well. This new device uses the principles of NIRAF imaging, however, it is able to overlay the projection of the PGs AF in the surgical field instead of a projection into a separate screen. PTeye (Medtronic, Minneapolis, MN, USA) was able to identify 98% of the PGs compared to a detection rate of 91% by experienced surgeons, all of this in the presence of ambient operating room lights, allowing a continuous surgical flow. On the other hand, OTIS was able to identify 97% of the PGs while providing true spatial context for the signal (23). Though this ground-breaking device is able to enhance PGs identification within the surgical field, the creators consider additional optimization is needed prior to be available for clinical use (24).
Does NIRAF decrease the rate of hypocalcemia after thyroidectomy?
While there have been a growing number of papers published in the past 10 years using NIRAF for PGs identification, most of the studies have focus on the feasibility of this technology but there is a relative paucity of prospective studies analyzing its actual benefit (8). Only a few studies have been published comparing the use of NIRAF to surgeon identification of PGs during thyroidectomy (25-27).
In the first study, the authors compared two time periods in where the use of NIRAF was adopted by a single surgeon (25). The primary outcome evaluated was hypocalcemia defined as a calcium level <8 mg/dL. In 93 patients who underwent total thyroidectomy with the use of NIRAF, hypocalcemia was found in 5.2% of the cases compared to 20.9% when NIRAF was not used (P<0.05). Even though the authors acknowledge the fact that a Hawthorne effect could explain the difference seen in these two time periods, they attempted to control this with a second group operated by a different surgeon. In fact, the rates of hypocalcemia in total thyroidectomies done by a second surgeon during the two time periods were not different to the first control group but they were statistically significant higher to the group where NIRAF was used.
Later on, a randomized controlled clinical trial was carried out comparing the rates of postoperative hypocalcemia between patients undergoing total thyroidectomy with and without the use of NIRAF (26). Using block-randomization in a 1:1 ratio with an estimated incidence of the primary outcome of 40%, 85 patients were included per group. The baseline characteristics did not differed between groups with the majority of patients undergoing surgery for cancer. In their results, the incidence of hypocalcemia (<8 mg/dL) was 8.2% in the NIRAF group compared to 16.5% in the control, nonetheless no statistical significance was achieved (P=0.103). In spite of this, a subgroup analysis accounting for hypocalcemia lower than 7.6 mg/dL showed statistically significant difference with 1.2% seen in the NIRAF group compared to 11.8% in the control (P=0.005).
Our institutional experience in the use of NIRAF during total thyroidectomy was unable to replicate these findings (27). One hundred patients in which NIRAF was routinely used during total thyroidectomy were compared to 200 consecutive patients operated without this novel technology. The procedures were done by the senior author with the adjunct of surgical loupes in all cases. Although the rate of incidental parathyroidectomy was higher in the control group (14% vs. 6%, P=0.04), this did not impact the patient outcomes in terms of transient hypocalcemia (6.5% vs. 5%, P=0.798) nor permanent hypocalcemia (0.5% vs. 0.0%, P=1.0).
These three studies are encouraging as it is possible that NIRAF can improve patient outcomes and reduce the rates of hypocalcemia after thyroidectomy. This can be especially valuable for junior surgeons as recent studies have demonstrated they have higher tissue misclassification rate of PGs (28). However, further studies are still needed to confirm these findings. Additionally, other limitations of NIRAF should be noted as well.
Limitations of the technology
As previously mentioned, the difficulty of localizing PGs is due to its small size, variable location and inconspicuous color which mandates training of the surgeon’s eye to achieve identification (7). NIRAF offers augmented visualization of the PGs based on its fluorescence properties but false positives can still arise (16). This false positive mandate the training of the surgeon to properly identify the AF of the PGs which brings some subjectivity to the technology. This can be particularly important with NIR imaging, as there is no direct fluorescence quantification. To aid with this matter, artificial intelligence models have been built to help identify PGs (29). Using machine learning on PGs NIR images, a model was created with a recall and precision of 90.5% and 95.7%, respectively. Other authors consider that the inclusion of artificial intelligence can further increase the benefits of NIRAF (30). In fact the use of deep learning methods for PG detection has been by other authors with similar findings (31,32).
Another limitation was the need to use this technology with the room lights off as white light can interfere with the reading. This need to shift between lights on and off can theoretical slowdown the surgical flow, however newer systems have bypassed this restriction. As discussed before, the PTeye (Medtronic, Minneapolis, MN, USA) has the advantage of working with the operating room lights on (23). On the other hand, this device works by contact necessitating the tissue to be at least partially dissected in able to provide a signal and the surgeon needs to at least point to the area of interest. Fluobeam (Fluoptics, Grenoble, France) offers a more wide evaluation of the surgical field but the tissue penetration of NIR is about 3 mm and deeper structures will not be clearly recognized (5). In addition, the use of a separate screen can contribute to a mismatch of the surgical field with the NIRAF image, which would theoretically be solve once OTIS is available (23,24).
As mentioned before, a limitation of NIRAF is that it fluorescence persist ex vivo limiting the evaluation of PGs perfusion. Nevertheless, new studies have reported the use of imager splitter with a software processor to aid in the identification of PGs as well as dye-free angiography to evaluate its perfusion in four patients (33). These preliminary results suggest that dye-free angiography is possible. In a similar way, another group used laser speckle contrast imaging (LSCI), which allows for real-time superficial flow assessment, to evaluate parathyroid perfusion (34). In their study, LSCI values were able to differentiate hypocalcemia in 72 patients undergoing thyroidectomy.
As newer cameras seem to increase the detection rate of PGs compared to older generations, it is likely that in the future more studies will demonstrate the benefit of NIRAF during thyroidectomy and parathyroidectomy (35). The compilation of evidence suggests that NIRAF appears to be helpful for identification and preservation of PGs during thyroidectomy at this time (36). Even though incidental parathyroidectomy is lower in the hands of experience surgeons, this technology could furthermore decrease its rate. On the other hand, some evidence also suggests that NIRAF utility is intensified when used by junior surgeons (37).
Conclusions
Parathyroid tissue has an intrinsic fluorophore which emits AF while stimulated with NIR light. The feasibility of NIRAF for intraoperative PGs identification has been demonstrated with different technologies. It appears that NIRAF can improve the rates of hypocalcemia after thyroidectomy, however, further studies are needed to confirm this finding. Further studies in this area should also include cost analysis as well as comparison between the available devices.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ralph P. Tufano and Salem I. Noureldine) for the series “Novel Technology and Techniques in the Management of Thyroid Nodules” published in Annals of Thyroid. The article has undergone external peer review.
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-22-31/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-22-31/coif). The series “Novel Technology and Techniques in the Management of Thyroid Nodules” was commissioned by the editorial office without any funding or sponsorship. EB has consulting agreements with Aesculap, Ethicon and Medtronic. He has received honoraria for consulting work unrelated to this study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Olson E, Wintheiser G, Wolfe KM, et al. Epidemiology of Thyroid Cancer: A Review of the National Cancer Database, 2000-2013. Cureus 2019;11:e4127. [Crossref] [PubMed]
- Norenstedt S, Ekbom A, Brandt L, et al. Postoperative mortality in parathyroid surgery in Sweden during five decades: improved outcome despite older patients. Eur J Endocrinol 2009;160:295-9. [Crossref] [PubMed]
- Astl J, Plzák J, Laštůvka P, et al. Morbidity and mortality associated with thyroid surgery - retrospective analysis 19912010. Rozhl Chir 2021;100:118-25. [PubMed]
- Chen Z, Zhao Q, Du J, et al. Risk factors for postoperative hypocalcaemia after thyroidectomy: A systematic review and meta-analysis. J Int Med Res 2021;49:300060521996911. [Crossref] [PubMed]
- Falco J, Dip F, Quadri P, et al. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg 2016;223:374-80. [Crossref] [PubMed]
- Adam MA, Thomas S, Youngwirth L, et al. Is There a Minimum Number of Thyroidectomies a Surgeon Should Perform to Optimize Patient Outcomes? Ann Surg 2017;265:402-7. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014;99:4574-80. [Crossref] [PubMed]
- Abbaci M, De Leeuw F, Breuskin I, et al. Parathyroid gland management using optical technologies during thyroidectomy or parathyroidectomy: A systematic review. Oral Oncol 2018;87:186-96. [Crossref] [PubMed]
- Sliney DH. What is light? The visible spectrum and beyond. Eye (Lond) 2016;30:222-9. [Crossref] [PubMed]
- du Plessis AJ. Near-infrared spectroscopy for the in vivo study of cerebral hemodynamics and oxygenation. Curr Opin Pediatr 1995;7:632-9. [PubMed]
- Das K, Stone N, Kendall C, et al. Raman spectroscopy of parathyroid tissue pathology. Lasers Med Sci 2006;21:192-7. [Crossref] [PubMed]
- Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013;154:1371-7; discussion 1377. [Crossref] [PubMed]
- McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016;159:193-202. [Crossref] [PubMed]
- Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab 2016;101:4646-52. [Crossref] [PubMed]
- De Leeuw F, Breuskin I, Abbaci M, et al. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J Surg 2016;40:2131-8. [Crossref] [PubMed]
- Kahramangil B, Dip F, Benmiloud F, et al. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Ann Surg Oncol 2018;25:957-62. [Crossref] [PubMed]
- Ladurner R, Sommerey S, Arabi NA, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017;31:3140-5. [Crossref] [PubMed]
- Ladurner R, Al Arabi N, Guendogar U, et al. Near-infrared autofluorescence imaging to detect parathyroid glands in thyroid surgery. Ann R Coll Surg Engl 2018;100:33-6. [Crossref] [PubMed]
- Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. [Crossref] [PubMed]
- Zaidi N, Bucak E, Okoh A, et al. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 2016;113:771-4. [Crossref] [PubMed]
- Berber E, Akbulut S. Can near-infrared autofluorescence imaging be used for intraoperative confirmation of parathyroid tissue? J Surg Oncol 2021;124:1008-13. [Crossref] [PubMed]
- Thomas G, McWade MA, Nguyen JQ, et al. Innovative surgical guidance for label-free real-time parathyroid identification. Surgery 2019;165:114-23. [Crossref] [PubMed]
- McWade MA, Thomas G, Nguyen JQ, et al. Enhancing Parathyroid Gland Visualization Using a Near Infrared Fluorescence-Based Overlay Imaging System. J Am Coll Surg 2019;228:730-43. [Crossref] [PubMed]
- Benmiloud F, Rebaudet S, Varoquaux A, et al. Impact of autofluorescence-based identification of parathyroids during total thyroidectomy on postoperative hypocalcemia: a before and after controlled study. Surgery 2018;163:23-30. [Crossref] [PubMed]
- Dip F, Falco J, Verna S, et al. Randomized Controlled Trial Comparing White Light with Near-Infrared Autofluorescence for Parathyroid Gland Identification During Total Thyroidectomy. J Am Coll Surg 2019;228:744-51. [Crossref] [PubMed]
- Kim YS, Erten O, Kahramangil B, et al. The impact of near infrared fluorescence imaging on parathyroid function after total thyroidectomy. J Surg Oncol 2020;122:973-9. [Crossref] [PubMed]
- Thomas G, Solórzano CC, Baregamian N, et al. Comparing intraoperative parathyroid identification based on surgeon experience versus near infrared autofluorescence detection - A surgeon-blinded multi-centric study. Am J Surg 2021;222:944-51. [Crossref] [PubMed]
- Avci SN, Isiktas G, Berber E. A Visual Deep Learning Model to Localize Parathyroid-Specific Autofluorescence on Near-Infrared Imaging: Localization of Parathyroid Autofluorescence with Deep Learning. Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Duh QY, Davis SN. Autofluorescence and Artificial Intelligence: The Future of Parathyroid Surgery? Ann Surg Oncol 2022; Epub ahead of print. [Crossref] [PubMed]
- Apostolopoulos ID, Papandrianos NI, Papageorgiou EI, et al. Artificial Intelligence Methods for Identifying and Localizing Abnormal Parathyroid Glands: A Review Study. MAKE 2022;4:814-26. [Crossref]
- Kim Y, Lee HC, Kim J, et al. A coaxial excitation, dual-red-green-blue/near-infrared paired imaging system toward computer-aided detection of parathyroid glands in situ and ex vivo. J Biophotonics 2022;15:e202200008. [Crossref] [PubMed]
- Oh E, Lee HC, Kim Y, et al. A pilot feasibility study to assess vascularity and perfusion of parathyroid glands using a portable hand-held imager. Lasers Surg Med 2022;54:399-406. [Crossref] [PubMed]
- Mannoh EA, Thomas G, Baregamian N, et al. Assessing Intraoperative Laser Speckle Contrast Imaging of Parathyroid Glands in Relation to Total Thyroidectomy Patient Outcomes. Thyroid 2021;31:1558-65. [Crossref] [PubMed]
- Akbulut S, Erten O, Gokceimam M, et al. Intraoperative near-infrared imaging of parathyroid glands: A comparison of first- and second-generation technologies. J Surg Oncol 2021;123:866-71. [Crossref] [PubMed]
- Solórzano CC, Thomas G, Berber E, et al. Current state of intraoperative use of near infrared fluorescence for parathyroid identification and preservation. Surgery 2021;169:868-78. [Crossref] [PubMed]
- Kose E, Rudin AV, Kahramangil B, et al. Autofluorescence imaging of parathyroid glands: An assessment of potential indications. Surgery 2020;167:173-9. [Crossref] [PubMed]
Cite this article as: Romero-Velez G, Elshamy M, Berber E. A review of parathyroid autofluorescence imaging in detection and preservation of parathyroid glands during thyroid surgery. Ann Thyroid 2023;8:5.