
Laser ablation for thyroid nodules has come to age—a review
Background
The widespread use of thyroid ultrasonography resulted in a higher detection of thyroid nodules in adult population. Currently, the cytological diagnosis provided by ultrasound-guided fine-needle aspiration (US-FNA), an easy noninvasive procedure, prevent the need for diagnostic surgery and the majority of patients may be followed-up (1-8). As most thyroid nodules are asymptomatic, and their volume stays approximately stable over the course of time, after a benign cytological diagnosis the majority of them go to observation without therapy (1). However, some nodules (about 10%), mainly the larger ones at diagnosis, gradually increase their dimensions, causing local discomfort or compressive symptoms (9). After a generally protracted follow-up, these steadily growing nodules are eventually managed with thyroidectomy (2,3). Thyroid surgery is an invasive and expensive treatment and is followed by cosmetic damage due to the cervical scar—unless more complicated surgical approaches are employed (4-7,10)—and by the frequent necessity of substitution therapy (8,9). A feasibility study published in 2000, after a series of tests on experimental models, demonstrated that US-guided thermal ablation [laser ablation (LA)] with a laser source could be easily performed on thyroid lesions with minimal discomfort and risk for the patient (11). During the following 20 years, ultrasound (US)-guided ablation techniques performed on the thyroid gland by means of different energy sources [besides LA, radiofrequency ablation (RFA), microwave ablation (MWA), and high-intensity focused ultrasound (HIFU)] (12,13) have been proposed and are currently used in routine clinical practice. Amidst these thermal procedures, LA may be easier to learn and represents a reliable and efficient procedure for the treatment of benign and selected malignant thyroid lesions.
Technique
Laser basic principles
Laser is a coherent and monochromatic light beam that can be sharply focused on an identified area, allowing the conveyance of light energy at a distance using optical fibers. The use of interstitial laser coagulation for pathologic human tissues was initially proposed by Bown in 1983, for the phototherapy of skin tumors and metastasis (14), and was subsequently confirmed as an affective ablation modality in malignant liver tumors (15). The optical fibers are thin and flexible devices, with a 300–600 µm diameter quartz optical fiber with flat-tip, that can efficiently transmit laser light to their extremities. When laser light hits organic tissues, scattering and absorption of the photons take place, with energy transfer, rapid heating of the tissue, and progressive thermal injury (11,16-18).
Various laser sources and wavelengths are usable and several kinds of fibers and applicators may be utilized. At present, in most of medical procedures, either Nd:YAG (operating at 1,064 nm) or diode lasers (820 nm), with no demonstrated differences in outcomes, working at an output range of 2–20 W are used (17). These sources provide effective energy penetration and absorption, especially in deep-seated and blood perfused organs.
Thermal energy increases tissue temperature and an irreversible cellular damage starts when tissue reaches a temperature of at least 46 °C for 60 minutes. The increase of the temperature level between 60 and 100 °C results in an increasingly fast coagulation of proteins, enzymes, and nucleic acids, that is followed in a few days by cellular destruction (14,16,19-24). In different tissues a variable thermal energy is required to induce cell death (25), but temperatures greater than 100 °C should not be attained because they result in tissue vaporization and carbonization, changes that may hinder a complete ablation of the target due to their insulating effect on energy diffusion (26-30). Laser thermal ablation (LTA) may induce in human tissues an area of coagulative necrosis with predictable size and geometrically defined margins. In comparison to the other available thermal ablation techniques, LA delivers less total energy in a well circumscribed area, which may grant greater control and safety in critical areas, while still achieving target temperature useful to obtain the irreversible damage of the tissue (31).
Procedure
LA is always performed under US guidance. Based on specific circumstances, the procedure may be US-guided (with the use of a dedicated device connected to US probe, helping in predetermine the trajectory of the fiber inside the neck) or US-assistance (with a free-hand technique), by means of an US system equipped with a high-frequency linear transducer (7.5–15 MHz) and combined with a planning and simulation software (Figure 1). The applicator used for the insertion of the fiberoptic in the target lesion is generally the lean and flexible, minimally traumatizing, 21-G spinal needle that ensures an accurate and cautious placement of one or more fibers (32).
The procedure is typically performed on the patient laying supine with hyperextended neck. The patients and operators’ eyes should be protected with glasses to prevent inadvertent injury. The skin should be cleansed, and the field draped. Local anesthesia is administered at the skin entry site, along the planned insertion tract, and around the thyroid capsule. Mild sedation with benzodiazepine is generally appropriate but, in case of anxious subjects, conscious sedation with intravenous midazolam may be performed. However, it is desirable that patients remain awake and able to speak, complaining at occurrence in case of severe local pain and to answering in case questions from the operator (32).
Under US monitoring, a 21-G, 2-inch-long needle is introduced into the target area, either with a trans-isthmic or a longitudinal, along the longest axis, approach. Pre-operative US should assess the anatomic landmarks for the choice of the appropriate approach. Among them, the tracheoesophageal groove, where the recurrent laryngeal nerve (RLN) is supposed to run, the anterior jugular veins, the vagus nerve in the carotid sheath, and the area of the sympathetic chain. Procedures are usually performed with a fixed-power protocol (3–5 W). Differently, the energy-delivering time (illumination time) varies according to the volume to be ablated. The typical illumination time ranges from 400 to 600 seconds, with each optical fiber delivering a total energy of 1,200–1,800 J (33).
When multiple applicators, two in most circumstances, are used, they are placed on an equal plane and should be spaced by 8 mm to grant the synchronous control of fibers (Figure 1 and Figure 2A). Due to the forward direction of laser illumination, the fiber tips should be placed with a safe distancing space of 5–10 mm from the thyroid capsule and the dangerous structures of the neck. Hydro-dissection using saline solution injection—that appears as an anechoic band separating the area to be ablated from the surrounding structures—should be performed to insulate the focus area in case it is near to carotid vessels or airway, as for cancer recurrences or disease persistence in the thyroid bed. Up to three illuminations (intended as a delivery of 1,200–1,800 J from a single fiber) may be performed during the same procedure by each fiber, drawing it back by 10 mm with a “pullback” technique at competition of each illumination time. In case of patient’s complain, the fiberoptic closer to the thyroid capsule should be shut-off and retracted before restarting the treatment (33).
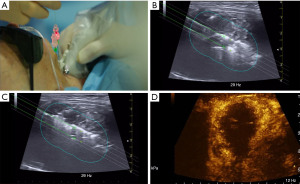
US monitoring reveals in the treated area the appearance of a hyperechoic zone - due to microbubbles produced by tissue water evaporation—that progressively enlarges during the illumination (Figure 2B,2C). When the area of hyper echogenicity is stationary in size the ablation treatment is ended, but the volume of coagulative necrosis attains its maximum size 48–72 hours later due to the thrombosis of the small vessels that supply the tissue (18,33-39).
US evaluation following treatment helps in identifying not ablated areas that can be retreated if appropriate. In this context, contrast-enhanced US may increase the quality of the evaluation of the disappearance of small vessel signals and so defining a not completely ablated zone (40) (Figure 2D). After neck dressing, the application of an ice pack decreases local edema and discomfort. An anti-inflammatory medication may be given parenterally, followed by analgesics by mouth during the following 24–48 hours. Patients should be observed for 60 minutes prior to discharge. Antibiotics are indicated only in the rare cases of infection.
Pre-operative assessment
For benign thyroid nodules, the volume of the nodule represents an objective criterion for the indication to treatment. However, neck circumference and nodule location within the gland may strongly influence the relevance of symptoms and of cosmetic concern (20). Current guidelines suggest a possible minimum diameter of 20–30 mm (20,41). Besides the objective size criteria, the presence of local pressure symptoms, progressive growth, and cosmetic concern may be considered as factors that strengthen the indication to LA. If suspected, retrosternal extension of large nodules should be evaluated with cross-sectional imaging, because a relevant immersion in the anterior mediastinum makes the ablation procedure technically cumbersome (13,32,42).
Hyperfunctioning thyroid nodules may also represent an indication to thermal ablation, but the control of hyperthyroidism is less predictable than after traditional treatment with radioactive iodine (RAI) or lobectomy (36,40,43-45). Because long-term control of hyperfunction is reported as associated with a nodule volume reduction of at least 80% and with the milder forms of hyperthyroidism, laser treatment is mainly indicated for patients with relatively small hyperfunctioning nodules (e.g., less than 3 cm in major diameter), who prefer to preserve an intact thyroid function, or with contraindications to RAI or surgery (40).
A benign cytologic diagnosis must be confirmed with two US-guided FNA prior to thermal ablation. When ultrasonographic features of the thyroid nodule are highly suspicious for benignity (e.g., spongiform and purely cystic nodules), the second fine-needle aspiration biopsy (FNAB) may be omitted. Cytologic assessment is not needed in hyperfunctioning nodules proved by scintigraphy (13,32,42). In cytologically benign nodules with suspicious US features, further clinical and cytological evaluation should be performed to rule out the risk of overlooking a malignant lesion. Treatment of cytologically indeterminate nodules (Bethesda III and IV categories) with laser appears inappropriate. In case of malignancy, thermal ablation of the target lesion may be oncologically incomplete and is associated with the risk of tumor progression, locally or at distance (13).
Mixed or predominantly cystic lesions are effectively treated with US-guided ethanol injection, a simple, safe, and inexpensive procedure. LA, however, may be performed immediately after fluid drainage in cystic nodules that recurred after ethanol injection (42).
Unless the patient is at high risk of cardiovascular events, discontinuation of antiplatelet therapy for 5 days prior to the procedure is suggested. The anticoagulant treatment with direct oral anticoagulants should be withdrawn for 24–36 hours, while for patients on warfarin treatment the INR should be less than 3. Antiplatelet and anticoagulant treatment can be safely resumed 24 hours after completing the procedure (13,42).
Laryngoscopy is routinely recommended in patient with relevant prior neck surgical history or with any relevant voice impairment assessment. In any other case, laryngoscopy is not necessary but suggested, since the simple voice assessment through anamnesis and clinical evaluation alone is not sufficient (13). Importantly, the patient should be made aware of the modalities of laser treatment, its expected outcomes, the possible management alternatives, and the need of US follow-up for a timely assessment of potential regrowth and need of repeat intervention. An informed consent to the procedure should be signed (13,42).
Clinical outcomes
Benign thyroid nodules
The feasibility study on US-guided thermal ablation for the treatment of thyroid nodules in humans was published in 2000 in Radiology (11) after a series of experimental tests during the 1990s, first on liver tissue, then on animal models, and finally ex vivo on freshly resected thyroid glands (46). The predictable and geometrical shape of tissue destruction and its correlation with the amount of delivered energy validated the data formerly obtained by the experimental models (47). Further confirmation was provided in 2012 by a larger study on 22 thyroid nodules that were surgically removed 1 after later laser thermal treatment. Surgical specimens systematically documented a clear-cut areas of coagulative necrosis surrounded by inflammatory response (35).
Since then, a number of case reports (36,48) non-randomized (22-24,37,47) and randomized trials (28,33,49,50) have addressed the use of LA for non-surgical management of thyroid lesions. All these trials confirmed the clinical efficacy, safety, and reproducibility in different centers of laser treatment for thyroid nodular disease. As a whole, 12 months after LA the nodule volume decrease ranged from 43% to 84% versus the baseline value (38,50). Long-term follow-up demonstrates that the nodule shrinkage is generally persistent because the mean nodule volume reduction was 48% and 51%, compared to pre-treatment, at 3-year (34) and 5-year (51) at the US assessment. Nodule volume reduction between 49% and 60% after 3 years from LA was observed in another multicenter prospective randomized trial, evaluating performances of single ablative procedures using fixed parameters (33). Interestingly, less than 10% of the treated nodules demonstrated an initial regrowth 3 years after treatment, with an increased probability in largest volume lesions (34). The relationship of the volume reduction with the initial size and with the US structure of the nodules—spongiform nodules performing better than solid compact lesions—was confirmed by subsequent studies. These data had a real-world confirmation by an externally monitored multicenter retrospective study. The re-evaluation of 1,531 patients treated during routine clinical activity (39) demonstrated a 60–80% decrease of the volume of thyroid nodules. The number of treatments ranged from one to three in relation to the initial size and the clinical response of the target lesions. Finally, a recent retrospective study, including 171 patients treated with a single LA, demonstrated that the volume reduction rate was stable at 59% after 10 years of follow-up. When patients were grouped in accordance with baseline nodule volume (<15, 15–25, or >25 mL), the largest, most prolonged effect was observed in patients with nodules <15 mL (41).
The decrease in the size of the nodules is paralleled by the improvement of local symptoms and cosmetic concern. Nearly in all patients that underwent LA, the disappearance or relevant amelioration of local compression symptoms or esthetic concern were reported using a visual-analogue questionnaire, defining the treatment as well tolerated (28,33,50,51). A prospective study was specifically addressed to assess the changes in QoL in patients treated with LA through the validated 13-scale ThyPRO questionnaire. After LA, visual analog scale (VAS) scores improved significantly from 1 week onwards in 100% of patients, while a significant improvement was seen in the goiter symptoms score after 1 month and in the general score and mean values of ThyPRO after 6 months (52).
The occurrence of minor and major complications is definitely low. Besides the low incidence of complications reported in prospective trials (30,38,50,52), the real-world study that collected 1,531 thyroid lesions demonstrated a 0.5% rate for both minor and major complications (39). These data were confirmed by a later, and extremely large, series of 2,345 unselected patients treated with LA that reported a 0.7% and 1.4% rate of major and minor complications, respectively. The complication rate is strictly related to the operator’s expertise. Up to now, the single case of tracheal damage that required surgical intervention occurred to an inexperienced physician (53) and, in a series of 122 thermal ablation performed by operators at the beginning of their learning curve, RLN damage, ether temporary or persistent, was recorded in 1.6% of patients (34). On the other hand, a trial involving four centers with particular experience in minimally invasive procedures reported a lower risk of minor complications, with occurrence of spontaneously resolving vocal cord paresis in less than 1% of treated cases (33).
The clinical outcomes and main characteristics of the relevant studies on this issue are summarized in Table 1.
Table 1
| Author | Patients/nodules No. | RCT | Baseline volume (mL), mean | Laser source | Major complication, n (%) | Minor complication, n (%) | Follow-up (months) | Volume reduction (%), mean |
|---|---|---|---|---|---|---|---|---|
| Døssing et al. (32) | 16 | No | 10.0 | 820 diode | 0 | 0 | 6 | 46 |
| Spiezia et al. (49) | 5/5 | No | 11.1 | Nd:YAG | 1 (8.3)a | 0 | 12 | 61 |
| Pacella et al. (11) | 8/8 | No | 22.7 | Nd:YAG | 2 (8.3)b | 0 | 6 | 63 |
| Papini et al. (54) | 20 vs. 20c | No | 24.1 | Nd:YAG | 0 | 0 | 6 | 64 |
| Døssing et al. (36) | 15 vs. 15d | Yes | 8.2 | 820 diode | 0 | 0 | 6 | 44* |
| Døssing et al. (37) | 10/10 | No | 9.6 | 820 diode | 0 | 0 | 12 | 57* |
| Døssing et al. (37) | 15 vs. 15d | Yes | 10.1/10.7 | 820 diode | 0 | 0 | 6 | 45 vs. 58* |
| Gambelunghe et al. (55) | 5 vs. 5c | Yes | 8.2 | Nd:YAG | 0 | 0 | 30 weeks | 44 |
| Cakir et al. (33) | 15/12 | No | 11.9 | 810 diode | 0 | 1 (7.6) | 12 | 82 |
| Papini et al. (44) | 21 vs. 21 vs. 20e | Yes | 11.7/13.6/12.1 | Nd:YAG | 0 | 0 | 12 | >40 |
| Valcavi et al. (43) | 122/122 | No | 23.1 | Nd:YAG | 3 (2.5) | 20 (16.3) | 36 | 48 |
| Døssing et al. (56) | 78/78 | Yes | 8.2 | 820 diode | 0 | 0 | 67 | 51* |
| Amabile et al. (34) | 51/51 | No | 53.5 | 980 diode | 0 | 0 | 12 | 82 |
| Gambelunghe et al. (57) | 20 vs. 20f | No | 15/14 | Nd:YAG | 0 | 2 (5) | 36 | +11/57 |
| Gambelunghe et al. (57) | 50 vs. 50g | No | 21/21 | Nd:YAG | 0–1 (0.02)a | 0 | 6 | 55/56* |
| Papini et al. (45) | 101 vs. 99h | Yes | 12 | Nd:YAG | 1 (1.0)a | 0 | 36 | 57 |
| Achille et al. (50) | 45/45 | No | 24 | Nd:YAG | 1 (2.5)a | 0 | 12 | 84 |
| Pacella et al. (51) | 1,531/1,534 | No | 27 | Nd:YAG | 8 (0.5) | 9 (0.6) | 12 | 73 |
| Negro et al. (58) | 56/56 | No | 15.7 | Nd:YAG | 4 (7.1)a | 0 | 48 | 56 |
| Mauri et al. (59) | 31/31 | No | 20.3 | Nd:YAG | 1 (0.02)a | 0 | 12 | 70 |
| Pacella et al. (60) | 449/449 | No | 21.5 | Nd:YAG | – | – | 12 | 63 |
| Oddo et al. (61) | 14/14 | No | 19 | Nd:YAG | 0 | 1 (7.1) | 12 | 58 |
| Rahal Junior et al. (62) | 30/31 | No | 12.44 | Nd:YAG | 0 | 10% | 12 | 60 |
| Døssing et al. (38) | 110 | No | 9 | 820 diode | – | – | 45 | 86 |
| Bernardi et al. (63) | 190 | No | 12.2 | Nd:YAG | – | – | 60 | 57 |
| Gambelunge et al. (64) | 171/171 | No | 16.0 | Nd:YAG | 0 | 27 (15.7) | 120 | 59 |
| Cesareo et al. (65) | 30/30 | Yes | 25.1 | Nd:YAG | 0 | – | 12 | 60 |
| Gambelunghe et al. (66) | 24 | No | 138 | Nd:YAG | 0 | 5 (20.0) | 48 | 80 |
*, median; a, transient dysphonia; b, transient dysphonia lasting 24 and 48 h, respectively; c, single LTA session vs. observation; d, one laser session vs. radioiodine therapy; e, LTA vs. L-T4 vs. observation; f, low amount of energy vs high amount of energy; g, patients treated with local anesthetic vs. patients treated without local anesthetic; h, 101 pts treated with LTA vs. 99 under observation. LTA, laser thermal ablation; L-T4, levo-thyroxine; pts, patients; RCT, randomized controlled trial.
Hyperfunctioning thyroid nodules
Several case reports and small series of patients demonstrated that LA treatment of hyperfunctioning thyroid nodules can restore normal thyroid function and may result in the disappearing of hypercaptation areas at thyroid scintigraphy (37,48,67). LA, however, induces the normalization of serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) levels only in a part of the patients and repeated laser treatments may be needed to achieve a persistent normalization of biochemical hyperthyroidism (36,43,47). These findings were confirmed by a prospective randomized trial that tested the efficacy of LA versus radioiodine treatment in 30 patients with overt hyperthyroidism due to functioning nodules (44). Patients underwent therapeutic radioiodine dose or alternatively a single procedure of thermal ablation. LA and radioiodine therapy induced, as a mean, a similar nodule volume reduction but LA induced less frequently than radioiodine the control of biochemical hyperthyroidism with TSH normalization in only 50% of the treated patients (44).
Relevant data for clinical practice were recently provided by a large, real life, multicenter study performed in Italy (40). A total of 361 patients, either treated with methimazole due to overt hyperthyroidism (70%) or with subclinical hyperthyroidism without any medical treatment (30%), were included. Nodules were treated using LA in 243/361 cases (67.3%) and RFA in 118 cases (32.7%) and were followed up to 3 years. The median volume reduction was 58% at 6-month and 60% at 12-month and the mean serum TSH values increased significantly at all time points. Importantly, anti-thyroid therapy was interrupted in 32.5% of patients at 2 months, in 38.9% at 6 months, and in 41.3% at 12 months. A significant difference in the rate of patients who withdraw medical therapy at 12 months was registered between small (<10 mL) (74%), medium (49%), or large (>30 mL) nodules (19%). Therapy was reduced, or withdrawn, with no significant distinction between the patients treated with the two different modalities of thermal ablation. A single major complication occurred (0.25%).
Thus, the available evidence demonstrates the clinical efficacy of LA only for the treatment of small size, solitary, and mildly hyperfunctioning nodules, not requiring o requiring low doses of anti-thyroid medication (43,45). As a persistent control of hyperthyroidism is achieved when nearly 80% of the hyperfunctioning nodule is ablated (68) clinical outcomes are expected as probably unsatisfactory in large size functional nodules and in those which require medium or high doses anti-thyroid medication. In these cases, the treatment with radioiodine or surgery should be preferred (36). LA, however, may be used as a combined treatment with radioiodine in large hyperfunctioning nodules which cause relevant pressure symptoms. In a prospective trial (69), 30 patients with a large hyperfunctioning nodule were randomly treated by LA followed by 131I or 131I alone. Data showed that combined treatments results in a faster nodule volume reduction and a faster amelioration of local symptoms than the therapy with radioiodine only.
Outcomes and main characteristics of the relevant studies on this issue are summarized in Table 2.
Table 2
| Author | Nodules No. | US pattern solidity ≥80 | Baseline volume (mL), mean | Lase source | Total energy load or J/mL, mean | Number of session, mean | Follow-up (months) | Volume reduction %, mean | Achieved euthyroidism |
|---|---|---|---|---|---|---|---|---|---|
| Døssing et al. (70) | 1 | Solid | 8.2 | 820 diode | 1,950 | 1 | 9 | 40 | 1/1 (100%) |
| Spiezia et al. (49) | 7 | Solid | 3.2 | Nd:YAG | Not reported | 1 | 12 | 74 | 1/7 (14%) |
| Pacella et al. (11) | 16 | Solid | 7.9 | Nd:YAG | 816 J/mL | 2.7 | 6 | 62 | 5/16 (31%) |
| Gambelunghe et al. (55) | 8 | Solid | 8.2 | Nd:YAG | 1,900 J/mL | 1 | 30 weeks | 44 | 8/8 (100%) |
| Døssing et al. (53)a | 14 vs. 15 | Solid | 10.6/11.2 | 820 diode | 217 J/mL | 1 | 6 | 44–47 | 7/14 (50%) vs. 15/15 (100%) |
| Valcavi et al. (71) | 1 | Solid | 2.5 | Nd:YAG | Not reported | 1 | Not reported | 95 | 1/1 (100%) |
| Rotondi et al. (48) | 1 | Solid | 55.0 | 980 diode | Not reported | 4 | 10 | 91 | 1/1 (100%) |
| Amabile et al. (34) | 26 | Solid | 55.3 | 980 diode | 379 J/mL | 3.2 cycle | 12 | 82 | 23/26 (88%) |
| Gambelunghe et al. (68) | 82 | Solid | 12 (5–118) | Nd:YAG | 7,200 J (median) | 1 | 12 | 42 | 65/82 (79%) |
a, single radioiodine dose vs. single laser therapy. US, ultrasound.
Cystic thyroid nodules
Since the long-established efficacy of US-guided ethanol injection, poorly data exists on the application of LA in cystic thyroid nodules (70), confirming the procedure as (39). After 6 months, a volume reduction >50% and the amelioration of local complains was registered in 15 of 22 (68%) of patients treated also with LA, against 4 of 22 (18%) patients in the aspiration-only group. LA resulted in volume reduction of solid component of the complex, predominantly cystic, nodules (from 1.8 to 1.0 mL) while the solid part was unchanged in the group treated with drainage only. LA was well tolerated, safe ed effective.
US-guided ethanol injection remains the first-line treatment for thyroid cysts and predominantly cystic nodules which relapse after initial drainage due to the low cost, safety, and efficacy of the procedure (55). Thus, LA may be appropriate for the management of cystic lesions recurring after percutaneous ethanol ablation to prevent persistent fluid refilling, and for mixed nodules with a not negligible solid portion, to achieve the shrinkage of the solid portion of the nodule (41).
The clinical outcomes and main characteristics of the relevant studies on this issue are summarized in Table 3.
Table 3
| Author | Nodules no. [pts] | US pattern solidity ≥80 | Baseline vol (mL), mean | Lase source | Follow-up (months) | Volume reduction (%), mean | Major complication, n (%) | Minor complication, n (%) |
|---|---|---|---|---|---|---|---|---|
| Døssing et al. (39) | 22 [22]a | Cystic | 11.8 | 820 diode | 36 | 57 | 0 | 0 |
a, aspiration followed by LTA session vs. aspiration without LTA. US, ultrasound; LTA, laser thermal ablation.
Papillary thyroid microcarcinoma (PTMC)
Background
During the last decades, the increased incidence of thyroid cancer—mostly due to US-guided FNA diagnosis of PTMC—has brought about the need for a tailored treatment of these tumors (56). Thus, due to the indolent nature of the majority of PTMC and the cost, risk, and impact on the quality of life of surgical procedures, active surveillance (AS) and minimally invasive procedures are currently proposed as alternative options to thyroidectomy for incidental PTMC (61,64,72).
Technical issues
Specific technical aspects are required for thermal ablation of primary thyroid tumors and of their recurrences. Laser treatment of unresectable tumors and of regional or distant metastasis is beyond the scope of this paper and we refer for these issues to our previous dedicated publications (12,13,31,73,74).
Oncologic treatment of PTMC requires the complete destruction of the target lesion and an extensive margin of tissue ablation of at least 2 mm is needed around the whole circumference of the tumor (13). Experimental and clinical data have demonstrated a correlation between the amount of laser energy delivered to the target lesion and the achieved volume of coagulative necrosis. With the distribution of 3,600 J, obtained with two optical fibers operating with a 3 W output for 600 seconds, an ablation volume of 5.3±1.6 mL (range, 5.0–8.0 mL) is achieved. Based on these parameters, the operator can plan the firing times and power output necessary for the complete PTMC eradication (31). Before treatment, the position of PTMC relative to the critical neck structures—including the RLN, esophagus, large vessels, and trachea—should be careful assessed. In these cases, the use of the hydrodissection technique is strongly needed for separating the target tumor from critical cervical structures with a liquid insulating band. Finally, at the end of the procedure, the optical fibers should be withdrawn while still illuminated to achieve the coagulation of the tracts and prevent risk of neoplastic seeding (31). Implantation of tumor cells in the subcutaneous layer or in ribbon-like muscles is reported in the literature: 0.14% of papillary thyroid carcinomas in one case series. It seems to be more likely in the case of aggressive or poorly differentiated variants (75).
Clinical outcomes
A case report reporting the use of LA for nonsurgical management of PTMC on an 81-year-old woman was published in 2011 (76). The patient had decompensated cirrhosis and had undergone recent surgery followed by external beam radiation for breast cancer. LA treatment of an 8-mm PTMC was followed by FNA and core needle biopsy (CNB) proven disappearance of viable malignant cells during a 2-year follow-up. In 2013, an experimental study demonstrated that laser treatment, performed on three PTMC before their surgical resection, resulted in the complete destruction of the neoplastic lesions at post-operative histology (77). A 12-month retrospective study on 30 patients with single PTMC demonstrated the US disappearance of the lesions in 33% of cases and the persistence of scar-like areas in the remaining. The complete disappearance of viable neoplastic cells was confirmed with repeat biopsy assessment in all cases (54).
Besides LA, RFA and MWA are increasingly used technologies for the ablation of PTMC (27,28,30,33,36-39,51). Due to the still limited number of cases, three systematic reviews and meta-analyses have assessed the combined efficacy and safety of RFA, MWA, and LA for this purpose. The first paper, evaluating 503 PTMCs, 8 to 53 months of monitoring, demonstrated neither local tumor recurrence nor distant metastases following thermal ablation (78). In 2 patients (0.4%) treated by LA, after 24 and 30 months of follow-up, metastases to cervical lymph node were detected. According to the international standardized terminology (76), no major complications were recorded, while from 3.4% to 19.0% of patients in different studies experienced minor complications. Another paper considered 715 PTMC monitored for 8 to 26 months after thermal ablation. The sonographic disappearance of the lesion was reported in 57.6% of treated lesions. Persistent scar-like zones, free of viable malignant cells at FNA and CNB examination, were observed in the remaining cases. Major complications (persistent dysphonia) were registered in 0.7% while minor complications occurred in 3.2% of cases, with a 0.4% local recurrence rate (77). A third, largest, meta-analysis, evaluated the outcomes of RFA, MWA, and LA in 1,284 PTMC. The results confirmed the elevated efficacy and substantial safety of thermal ablation treatment. Interestingly, among the different techniques, RFA obtained the highest disappearance rate (76.2%), and the lowest recurrence rate (0.01%), compared to other two techniques, although this trend did not reach the statistical significance. On the other hand, LA had the lower complication rate (0.9%) compared to RFA (1.7%) and MWA (6.0%), so confirming the minimally traumatic effects and the high precision of LA (79). These systematic review and meta-analysis demonstrated the consistent efficacy and safety of thermal ablation treatment for PTMC but also presented methodological limitations. They included both prospective and retrospective studies, their follow-up was generally too short for ruling out the slow PTMC progression, and the serum thyroglobulin and TSH levels were rarely considered.
Prospective randomized trials of LA versus surgery are lacking but comparative data from controlled retrospective studies are available. A series of 81 PTMC were treated with either surgery or LA and were followed up during a 4-year period (80). Nearly all (94.4%) ablated lesions entirely disappeared and 5.6% of them persisted as scar-like zones at US examination. Importantly, the complication rate was lower in the LA than in the surgery group (2.8% vs. 6.7%, respectively) while the recurrence rate, local or regional, did not differ significantly between the LA and the surgical group (5.6% vs. 6.7%, respectively). Notably, mean hospital stays and procedure time in LA group were 3.6±0.7 h and 25.9±7.0 min, respectively, which were much shorter than those of the surgical group (62.0±11.4 h and 74.2±12.5 min, respectively). Finally, the number of patients who needed thyroxine treatment during follow-up was significantly greater after surgery (100%) than that after LA treatment (52.8%) (80).
Based on these data, even if no head-to-head comparison of LA vs. AS is available, the potential benefit of LA of PTMC appears relevant. During long-term AS periods, a non-negligible minority of PTMC, especially in young patients, showed extra thyroid spread and cervical metastases (41,72,81). Clinically detectable lymph node metastasis during AS were detected in a minority of patients. Their occurrence ranged from 1.5% at 10 years (82) to 8.6% at 3 years (83).
Importantly, patients recruited in AS trials may undergo over time delayed surgery because of anxiety. Thus, the main benefit of LA, like for other thermal ablation techniques, is the opportunity of a treatment with an effectiveness similar to thyroidectomy, but devoid of the cost, risk, and impact on quality of life related to surgery (47).
Future perspectives
The available evidence on the clinical outcomes of LA is mostly based on short and medium term prospective randomized studies and on large retrospective series. Controlled head-to-head prospective trials of LA vs. surgery or radioiodine therapy are also lacking. Thus, long-term investigation is required to better define a few relevant areas, including the appropriate role of LA vs. surgery in cytologically benign thyroid nodules, the prognostic factors for successful ablation, and the appropriate indications of LA for PTMC and local cancer recurrences. Finally, the cost effectiveness for LA vs. the traditional surgical or radioiodine treatment is still to be established. The 2022 Italian National Guidelines for the management of symptomatic thyroid nodules defined the costs of surgical lobectomy and thyroid LA as high as €4.211 and €1.560, respectively. However, several variables, like the need of repeat treatments in case of nodule regrowth or the management of surgical complications and post-surgical hypothyroidism, may strongly influence this calculation.
Conclusions for clinical practice
The high prevalence of nodular thyroid disease in the general population represents a problem due to its burden on medical assistance and surgical procedures and the costs directly or indirectly generated. Thus, the management of benign and malignant thyroid nodules requires the availability of several therapeutic modalities to offer appropriate management options. Various US-guided thermal ablation techniques, with various action mechanisms, are now available as alternative to surgery. Unfortunately, a recent ETA survey demonstrated that thermal ablation procedures are considered as a possible option for thyroid nodules only by 16% of the European endocrinologists (12,13,32,42).
US-guided LA is the first proposed of the thermal ablation techniques for thyroid nodules, being developed on experimental and clinical grounds in 2000 starting for data of laser application on other organs. It may be safely used by physicians with experience in performing thyroid US examinations and US-guided fine needle aspiration. A dedicated training and an initial tutorship are needed for preventing the risk of complications, that lowers after 50 or more cases treated (84).
LA should be considered, for the management of symptomatic solid, or predominantly solid, non-functioning thyroid nodules. Two FNA assessments of the benign nature of the nodule are needed before treatment to rule out the risk of well-differentiated thyroid cancer. LA results in a clinically significant volume reduction, paralleled by amelioration of local complains in the majority of cases, up to 80% in the largest multicenter trial (39). Volume reduction is stable over many years, but LA should aim at the most complete ablation of the target nodule to prevent regrowth. Periprocedural complications are rare, the risks of cosmetic damage or thyroid function disorders are almost nonexistent, and the influence on quality of life is favorable. Several trials compared the outcomes of LA versus RFA and MWA. The results demonstrated a nearly similar efficacy of the three thermal ablation techniques. Therefore, the treatment modality should be chosen based on the competences and resources at the individual center.
Radioiodine therapy remains the treatment of choice of hyperfunctioning nodules because control of hyperthyroidism is obtained in over 90% of cases and the reduction of nodule volume ranges from 30% to 50% at 2 years (63). Advantages of radioiodine therapy are its low cost and ease of application as an outpatient procedure while the major disadvantage is the frequent (up to 60% at 20 years) development of hypothyroidism (85). Thus, LTA may be mainly considered for the management of small size hyperfunctioning thyroid nodules with subclinical hyperthyroidism. Particularly in young patients, thyroid function normalization is obtained with no irradiation or risk of late hypothyroidism.
Due to the efficacy, safety and minimal cost of US-guided ethanol injection, LA is a second-line treatment for cystic thyroid lesions which persistently relapse after ethanol ablation (86).
The use of LA and of the other minimally invasive techniques for non-surgical management of PTMC represents a rapidly evolving area. Currently, LA may be considered for patients with low-risk PTMC, mainly if they are surgically at risk, with short expectation of life, and if they refuse surgery or AS.
In all cases, before the procedure, the advantages and limitations of LA must be fully evaluated with the patient and weighted against the outcomes of the other management options.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ralph P. Tufano and Salem I. Noureldine) for the series “Novel Technology and Techniques in the Management of Thyroid Nodules” published in Annals of Thyroid. The article has undergone external peer review.
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-22-26/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-22-26/coif). The series “Novel Technology and Techniques in the Management of Thyroid Nodules” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab 1955;15:1270-80. [Crossref] [PubMed]
- Carroll BA. Asymptomatic thyroid nodules: incidental sonographic detection. AJR Am J Roentgenol 1982;138:499-501. [Crossref] [PubMed]
- Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer 1985;56:531-8. [Crossref] [PubMed]
- Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med 1998;17:487-96. [Crossref] [PubMed]
- Brander AE, Viikinkoski VP, Nickels JI, et al. Importance of thyroid abnormalities detected at US screening: a 5-year follow-up. Radiology 2000;215:801-6. [Crossref] [PubMed]
- Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005;237:794-800. [Crossref] [PubMed]
- Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am 2007;36:707-35. vi. [Crossref] [PubMed]
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Ann Intern Med 1997;126:226-31. [Crossref] [PubMed]
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA 2015;313:926-35. [Crossref] [PubMed]
- Aidan P, Arora A, Lorincz B, et al. Robotic Thyroid Surgery: Current Perspectives and Future Considerations. ORL J Otorhinolaryngol Relat Spec 2018;80:186-94. [Crossref] [PubMed]
- Pacella CM, Bizzarri G, Guglielmi R, et al. Thyroid tissue: US-guided percutaneous interstitial laser ablation-a feasibility study. Radiology 2000;217:673-7. [Crossref] [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Piccoli M, Mullineris B, Santi D, et al. Advances in Robotic Transaxillary Thyroidectomy in Europe. Curr Surg Rep 2017;5:17. [Crossref] [PubMed]
- Bown SG. Phototherapy in tumors. World J Surg 1983;7:700-9. [Crossref] [PubMed]
- Shan L, Liu J. A Systemic Review of Transoral Thyroidectomy. Surg Laparosc Endosc Percutan Tech 2018;28:135-8. [Crossref] [PubMed]
- Materazzi G, Fregoli L, Papini P, et al. Robot-Assisted Transaxillary Thyroidectomy (RATT): A Series Appraisal of More than 250 Cases from Europe. World J Surg 2018;42:1018-23. [Crossref] [PubMed]
- Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg 2008;393:667-73. [Crossref] [PubMed]
- Watt T, Hegedüs L, Groenvold M, et al. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol 2010;162:161-7. [Crossref] [PubMed]
- Mauri G, Hegedüs L, Bandula S, et al. European Thyroid Association and Cardiovascular and Interventional Radiological Society of Europe 2021 Clinical Practice Guideline for the Use of Minimally Invasive Treatments in Malignant Thyroid Lesions. Eur Thyroid J 2021;10:185-97. [Crossref] [PubMed]
- Orloff LA, Noel JE, Stack BC Jr, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck 2022;44:633-60. [Crossref] [PubMed]
- Vogl TJ, Müller PK, Hammerstingl R, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology 1995;196:257-65. [Crossref] [PubMed]
- Zervas NT, Kuwayama A. Pathological characteristics of experimental thermal lesions. Comparison of induction heating and radiofrequency electrocoagulation. J Neurosurg 1972;37:418-22. [Crossref] [PubMed]
- Goldberg SN, Gazelle GS, Compton CC, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000;88:2452-63. [Crossref] [PubMed]
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res 2005;127:208-23. [Crossref] [PubMed]
- Matthewson K, Coleridge-Smith P, O'Sullivan JP, et al. Biological effects of intrahepatic neodymium:yttrium-aluminum-garnet laser photocoagulation in rats. Gastroenterology 1987;93:550-7. [Crossref] [PubMed]
- Nikfarjam M, Malcontenti-Wilson C, Christophi C. Focal hyperthermia produces progressive tumor necrosis independent of the initial thermal effects. J Gastrointest Surg 2005;9:410-7. [Crossref] [PubMed]
- McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol 2001;176:3-16. [Crossref] [PubMed]
- McGahan JP, Browning PD, Brock JM, et al. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990;25:267-70. [Crossref] [PubMed]
- Heisterkamp J, van Hillegersberg R, IJzermans JN. Critical temperature and heating time for coagulation damage: implications for interstitial laser coagulation (ILC) of tumors. Lasers Surg Med 1999;25:257-62. [Crossref] [PubMed]
- Dachman AH, Smith MJ, Burris JA, et al. Interstitial laser ablation in experimental models and in clinical use. Seminars in Interventional Radiology 1993;10:101-12. [Crossref]
- Nolsøe CP, Torp-Pedersen S, Burcharth F, et al. Interstitial hyperthermia of colorectal liver metastases with a US-guided Nd-YAG laser with a diffuser tip: a pilot clinical study. Radiology 1993;187:333-7. [Crossref] [PubMed]
- Døssing H, Bennedbaek FN, Karstrup S, et al. Benign solitary solid cold thyroid nodules: US-guided interstitial laser photocoagulation--initial experience. Radiology 2002;225:53-7. [Crossref] [PubMed]
- Cakir B, Topaloglu O, Gul K, et al. Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest 2006;29:876-84. [Crossref] [PubMed]
- Amabile G, Rotondi M, Pirali B, et al. Interstitial laser photocoagulation for benign thyroid nodules: time to treat large nodules. Lasers Surg Med 2011;43:797-803. [Crossref] [PubMed]
- Pacella CM, Bizzarri G, Spiezia S, et al. Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 2004;232:272-80. [Crossref] [PubMed]
- Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules - a randomised study. Eur J Endocrinol 2005;152:341-5. [Crossref] [PubMed]
- Døssing H, Bennedbaek FN, Hegedüs L. Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules: one versus three treatments. Thyroid 2006;16:763-8. [Crossref] [PubMed]
- Døssing H, Bennedbæk FN, Hegedüs L. Long-term outcome following laser therapy of benign cystic-solid thyroid nodules. Endocr Connect 2019;8:846-52. [Crossref] [PubMed]
- Døssing H, Bennedbæk FN, Hegedüs L. Interstitial laser photocoagulation (ILP) of benign cystic thyroid nodules--a prospective randomized trial. J Clin Endocrinol Metab 2013;98:E1213-7. [Crossref] [PubMed]
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia 2019;36:376-82. [Crossref] [PubMed]
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J 2020;9:172-85. [Crossref] [PubMed]
- Pacella CM, Jiang T. Experimental data and clinical studies on laser ablation. In: Pacella C, Jiang T, Mauri G. editors. Image-guided Laser Ablation. Springer, Cham, 2020.
- Valcavi R, Riganti F, Bertani A, et al. Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 2010;20:1253-61. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bizzarri G, et al. Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 2007;17:229-35. [Crossref] [PubMed]
- Papini E, Rago T, Gambelunghe G, et al. Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 2014;99:3653-9. [Crossref] [PubMed]
- Piana S, Riganti F, Froio E, et al. Pathological findings of thyroid nodules after percutaneous laser ablation: a series of 22 cases with cyto-histological correlation. Endocr Pathol 2012;23:94-100. [Crossref] [PubMed]
- Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest 2010;33:1-50. [Crossref] [PubMed]
- Rotondi M, Amabile G, Leporati P, et al. Repeated laser thermal ablation of a large functioning thyroid nodule restores euthyroidism and ameliorates constrictive symptoms. J Clin Endocrinol Metab 2009;94:382-3. [Crossref] [PubMed]
- Spiezia S, Vitale G, Di Somma C, et al. Ultrasound-guided laser thermal ablation in the treatment of autonomous hyperfunctioning thyroid nodules and compressive nontoxic nodular goiter. Thyroid 2003;13:941-7. [Crossref] [PubMed]
- Achille G, Zizzi S, Di Stasio E, et al. Ultrasound-guided percutaneous laser ablation in treating symptomatic solid benign thyroid nodules: Our experience in 45 patients. Head Neck 2016;38:677-82. [Crossref] [PubMed]
- Pacella CM, Mauri G, Achille G, et al. Outcomes and Risk Factors for Complications of Laser Ablation for Thyroid Nodules: A Multicenter Study on 1531 Patients. J Clin Endocrinol Metab 2015;100:3903-10. [Crossref] [PubMed]
- Barbaro D, Orsini P, Lapi P, et al. Percutaneous laser ablation in the treatment of toxic and pretoxic nodular goiter. Endocr Pract 2007;13:30-6. [Crossref] [PubMed]
- Døssing H, Bennedbaek FN, Bonnema SJ, et al. Randomized prospective study comparing a single radioiodine dose and a single laser therapy session in autonomously functioning thyroid nodules. Eur J Endocrinol 2007;157:95-100. [Crossref] [PubMed]
- Papini E, Guglielmi R, Bizzarri G, et al. Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 2004;10:276-83. [Crossref] [PubMed]
- Gambelunghe G, Fatone C, Ranchelli A, et al. A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Invest 2006;29:RC23-6. [Crossref] [PubMed]
- Døssing H, Bennedbæk FN, Hegedüs L. Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 2011;165:123-8. [Crossref] [PubMed]
- Gambelunghe G, Bini V, Monacelli M, et al. The administration of anesthetic in the thyroid pericapsular region increases the possibility of side effects during percutaneous laser photocoagulation of thyroid nodules. Lasers Surg Med 2013;45:34-7. [Crossref] [PubMed]
- Negro R, Salem TM, Greco G. Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia 2016;32:822-8. [Crossref] [PubMed]
- Mauri G, Cova L, Monaco CG, et al. Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 2017;33:295-9. [Crossref] [PubMed]
- Pacella CM. Image-guided thermal ablation of benign thyroid nodules. J Ultrasound 2017;20:347-9. Erratum in: J Ultrasound 2018 Mar;21(1):79. [Crossref] [PubMed]
- Oddo S, Felix E, Mussap M, et al. Quality of Life in Patients Treated with Percutaneous Laser Ablation for Non-Functioning Benign Thyroid Nodules: A Prospective Single-Center Study. Korean J Radiol 2018;19:175-84. [Crossref] [PubMed]
- Rahal A Junior, Falsarella PM, Mendes GF, et al. Percutaneous laser ablation of benign thyroid nodules: a one year follow-up study. Einstein (Sao Paulo) 2018;16:eAO4279. [Crossref] [PubMed]
- Bernardi S, Giudici F, Cesareo R, et al. Five-Year Results of Radiofrequency and Laser Ablation of Benign Thyroid Nodules: A Multicenter Study from the Italian Minimally Invasive Treatments of the Thyroid Group. Thyroid 2020;30:1759-70. [Crossref] [PubMed]
- Gambelunghe G, Stefanetti E, Avenia N, et al. Percutaneous Ultrasound-Guided Laser Ablation of Benign Thyroid Nodules: Results of 10-Year Follow-Up in 171 Patients. J Endocr Soc 2021;5:bvab081. [Crossref] [PubMed]
- Cesareo R, Manfrini S, Pasqualini V, et al. Laser Ablation Versus Radiofrequency Ablation for Thyroid Nodules: 12-Month Results of a Randomized Trial (LARA II Study). J Clin Endocrinol Metab 2021;106:1692-701. [Crossref] [PubMed]
- Gambelunghe G, Ristagno S, Stefanetti E, et al. Ultrasound-guided laser ablation of very large benign thyroid nodules: 4-year, retrospective follow-up in 24 patients. Int J Hyperthermia 2022;39:217-21. [Crossref] [PubMed]
- Mauri G, Papini E, Bernardi S, et al. Image-guided thermal ablation in autonomously functioning thyroid nodules. A retrospective multicenter three-year follow-up study from the Italian Minimally Invasive Treatment of the Thyroid (MITT) Group. Eur Radiol 2022;32:1738-46. [Crossref] [PubMed]
- Gambelunghe G, Stefanetti E, Colella R, et al. A single session of laser ablation for toxic thyroid nodules: three-year follow-up results. Int J Hyperthermia 2018;34:631-5. [Crossref] [PubMed]
- Pacella CM, Rossi Z, Bizzarri G, Papini E, Marinozzi V, Paliotta D, et al. Ultrasound-guided percutaneous laser ablation of liver tissue in a rabbit model. Eur Radiol 1993;3:26-32. [Crossref]
- Døssing H, Bennedbaek FN, Hegedüs L. Ultrasound-guided interstitial laser photocoagulation of an autonomous thyroid nodule: the introduction of a novel alternative. Thyroid 2003;13:885-8. [Crossref] [PubMed]
- Valcavi R, Bertani A, Pesenti M, et al. Laser and radio- frequency ablation procedures. In: Baskin HJ, Duick DS, Levine RA, editors. Thyroid ultrasound and ultrasound guided FNA biopsy. 2nd edition. New York: Springer; 2008:191-218.
- Pacella CM, Mauri G. Is there a role for minimally invasive thermal ablations in the treatment of autonomously functioning thyroid nodules? Int J Hyperthermia 2018;34:636-8. [Crossref] [PubMed]
- Di Rienzo G, Surrente C, Lopez C, et al. Tracheal laceration after laser ablation of nodular goitre. Interact Cardiovasc Thorac Surg 2012;14:115-6. [Crossref] [PubMed]
- Cakir B, Gul K, Ugras S, et al. Percutaneous laser ablation of an autonomous thyroid nodule: effects on nodule size and histopathology of the nodule 2 years after the procedure. Thyroid 2008;18:803-5. [Crossref] [PubMed]
- Chianelli M, Bizzarri G, Todino V, et al. Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metab 2014;99:E1283-6. [Crossref] [PubMed]
- Papini E, Guglielmi R, Gharib H, et al. Ultrasound-guided laser ablation of incidental papillary thyroid microcarcinoma: a potential therapeutic approach in patients at surgical risk. Thyroid 2011;21:917-20. [Crossref] [PubMed]
- Valcavi R, Piana S, Bortolan GS, et al. Ultrasound-guided percutaneous laser ablation of papillary thyroid microcarcinoma: a feasibility study on three cases with pathological and immunohistochemical evaluation. Thyroid 2013;23:1578-82. [Crossref] [PubMed]
- Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:1856-83. [Crossref] [PubMed]
- Zhang L, Zhou W, Zhan W, et al. Percutaneous Laser Ablation of Unifocal Papillary Thyroid Microcarcinoma: Utility of Conventional Ultrasound and Contrast-Enhanced Ultrasound in Assessing Local Therapeutic Response. World J Surg 2018;42:2476-84. [Crossref] [PubMed]
- Cho SJ, Baek JH, Chung SR, et al. Thermal Ablation for Small Papillary Thyroid Cancer: A Systematic Review. Thyroid 2019;29:1774-83. [Crossref] [PubMed]
- Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014;273:241-60. [Crossref] [PubMed]
- Choi Y, Jung SL. Efficacy and Safety of Thermal Ablation Techniques for the Treatment of Primary Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis. Thyroid 2020;30:720-31. [Crossref] [PubMed]
- Tong M, Li S, Li Y, et al. Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019;36:1278-86. [Crossref] [PubMed]
- Zhou W, Ni X, Xu S, et al. Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia 2019;36:897-904. [Crossref] [PubMed]
- Jeon MJ, Kim WG, Chung KW, et al. Active Surveillance of Papillary Thyroid Microcarcinoma: Where Do We Stand? Eur Thyroid J 2019;8:298-306. [Crossref] [PubMed]
- Guglielmi R, Pacella CM, Bianchini A, et al. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid 2004;14:125-31. [Crossref] [PubMed]
Cite this article as: Papini E, Bizzarri G, Novizio R, Guglielmi R. Laser ablation for thyroid nodules has come to age—a review. Ann Thyroid 2023;8:4.


