
Radiofrequency ablation of benign and malignant thyroid nodules: updates and current status
Introduction
Thyroid nodules are exceedingly common, being detected in up to two-thirds of adults, and often discovered incidentally (1). Though the vast majority of nodules are benign, 7% to 15% harbor malignancy (2). Surgery, whether open or via remote access approaches, has been the traditional method to address thyroid malignancy as well as symptomatic benign disease. However, due to the indolent nature of most thyroid pathology and the potential for long term surgical consequences, the decisions regarding whether and when to pursue surgery can often prove complex. Though generally safe and well-tolerated, thyroid surgery does involve risk to the laryngeal nerves, parathyroid glands, and other critical structures. Additionally, even following lobectomy alone, lifelong thyroid hormone replacement may be necessary in up to 38% of patients with benign disease, and 84% with malignancy (3,4).
In recent years, image-guided ablative technologies have emerged as a less invasive therapeutic option that may limit or avoid risks often associated with surgery, while achieving durable volume reduction and symptomatic relief. Tissue destruction can be achieved via thermal injury, as with radiofrequency ablation (RFA), laser ablation (LA), microwave ablation (MWA), and high-intensity focused ultrasound (HIFU), or may be chemical in nature, as with ethanol ablation. While the various thermal technologies rely upon differing modalities of heat delivery, all achieve the common outcome of targeted ablation that protects critical structures and preserves normal thyroid function. In particular, RFA has become the most globally adopted thermal strategy (5), and is the focus of this review.
Principles of RFA
Thermal ablation of tissue is a sequence of protein denaturation, coagulative necrosis, and eventually vaporization that occurs at supraphysiologic temperatures. Above 60 °C, immediate cell death is caused by cellular membrane disruption and protein denaturation. Above 100 °C, water vaporization and tissue carbonization occur. This creates char and coagulum at the active tip of the probe, which impairs further ablation. Therefore, intermediate temperatures of 50–90 °C are targeted (6).
RFA relies on an alternating current at the active tip of a probe inserted under ultrasound guidance to generate heat via ionic friction, which then spreads into immediate surrounding tissue (7). Several factors influence the delivery of thermal injury, including: the heat sink effect mediated by blood flow within and surrounding the target tissue, the impedance of the tissue which resists the alternating current, and the ability of the target tissue to conduct thermal energy, which varies by density and water content (7). To assure safe treatment of the entire nodule, the trans-isthmic moving shot technique is employed (8). The active probe is introduced through the isthmus and positioned in the deepest portion of the nodule. Ablation proceeds as the tip is withdrawn and evidence of echogenic change is witnessed on ultrasound, as shown in Figure 1 (8).
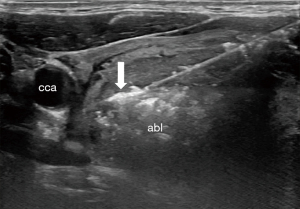
Benign thyroid nodules
RFA is indicated for patients with benign nodules causing compressive or cosmetic concerns, which, depending on location and patient habitus, are generally greater than 20–30 mm in size (9-11). Smaller nodules may rarely be candidates for ablation, but should directly correlate with patient reported symptoms. Consensus recommends against treating asymptomatic nodules, regardless of size (8). The preoperative workup of these patients should include laboratory testing for TSH, as well as two benign FNAs (8). A single biopsy may be adequate in cases where the nodule shows ultrasonographic features strongly associated with benignity, such as spongiform lesions (5).
Volume reduction of 50% is considered a procedural success, as it is often sufficient to relieve compressive symptoms and cosmetic concerns. Average volume reduction between 67% and 81% at 12 months are reported after a single session of RFA (12-14). Figure 2 demonstrates the typical appearance of a nodule treated with RFA and expected volumetric response. With multi-year follow up and repeat ablative sessions, average volume reduction exceeding 90% has been achieved in several studies (15-17). Nodules with initial volume <10 mL have been shown to achieve greater and more durable volume reduction (18). Patients, should, therefore, be aware of the potential necessity of two or more procedures to achieve desired outcomes depending on initial nodule size and treatment response. RFA can also be an effective option in the setting of prior thyroid surgery. In a study of 20 patients with history of previous thyroid lobectomy, RFA achieved reduction of 85.41% with no effect on pre-procedure thyroid hormone status (19). Bilateral nodules may also be treated effectively, though separate sessions are recommended to maximize ablative efficacy and safety (20).
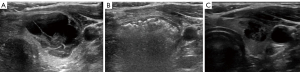
Additionally, the rate of complications in patients undergoing RFA for symptomatic thyroid nodules has been shown to be lower than in patients undergoing surgery (21,22). A primary advantage of RFA is the preservation of normal thyroid parenchyma, which results in the low risk of hypothyroidism in long term follow up (17). A 2014 study of 111 patients treated with RFA or lobectomy reported 23% of surgical patients required daily thyroid hormone, while no patients in the RFA group became hypothyroid post-procedurally (23). Risk factors for long-term post-RFA hypothyroidism include higher baseline thyroid stimulating hormone (TSH) levels and the presence of thyroid peroxidase antibodies (24). Recurrent laryngeal nerve paresis (8% versus 2.7%), and hypoparathyroidism (5.4% versus 0%) were also lower in the RFA group (23). Unique to RFA, nodule rupture may rarely occur weeks to months following RFA, and presents as a sudden painful neck swelling. Most cases of rupture may be treated conservatively (25). Overall, RFA is increasingly recognized as a valuable option for the patient with symptomatic or cosmetically bothersome benign thyroid nodules.
Autonomously functioning thyroid nodules (AFTN)
RFA has been suggested as an alternative therapy for AFTN in patients with overt or subclinical hyperthyroidism unwilling to undergo surgery or radioactive iodine. However, achievement of euthyroidism without medication is variable, and less reliable than with surgery or radioactive iodine. Bernardi et al. reported 50% of patients were able to discontinue anti-thyroid medication 12 months after a single session of RFA (26). Systematic review of RFA in AFTNs noted a pooled TSH normalization rate of 57% at 12 months (27). Success in treating AFTN is associated with a volume reduction of at least 80%, and thus smaller nodules may be more appropriately suited for monotherapy with RFA (28).
There may additionally be a role for a combination therapy involving RFA and radioactive iodine (RAI) ablation. Mader et al. reported a series of 15 patients receiving combination RFA and RAI (29). Volume reduction in this group was significantly improved compared with the RAI only group, and euthyroidism was achieved in all patients (29). Unpublished data from this same group suggests combination therapy may also require lower I131 doses. As opposed to benign nodule ablation, patients with AFTN may experience more significant changes in their post-procedure TSH, and require attentive post-procedural biochemical monitoring (26). Overall, as a euthyroid outcome is less certain with RFA than with standard therapeutic approaches, RFA is currently considered a second line option in patients unable or unwilling to undergo surgery or treatment with RAI (10,28).
Indeterminate nodules/follicular neoplasms
Literature concerning the role of ablative technologies in indetermination lesions is limited. Ha et al. reported excellent results in a series of 10 patients with follicular neoplasm <2 cm treated with RFA. Average volume reduction of 99.5% was achieved, and 8 nodules disappeared completely (30). Conversely, Dobrinja et al. performed RFA on 6 follicular neoplasms, 2 of which regrew and ultimately required surgery. Final surgical pathology revealed one follicular carcinoma and one follicular neoplasm of indeterminate malignant behavior (31). More recently, Lin et al. utilized PET/CT to determine candidacy for RFA in patients with follicular neoplasm. Patients with SUVmax ≥5 underwent surgical resection, while nodules with SUVmax <5 were treated with RFA. Volume reduction at 12 months in the RFA group was 73.3%, with no patient demonstrating regrowth or requiring surgery. In the surgery group were two Hurthle cell carcinomas, 2 follicular carcinomas, and two benign lesions (32).
Importantly, the distinction of adenoma from carcinoma in thyroid follicular lesions is based upon the presence of capsular or vascular invasion, which can only be determined following surgical excision. RFA and other ablative options do not permit definitive diagnostic maneuvers, and so are not currently considered first-line therapies for indeterminate lesions. The simultaneous acceleration of thyroid molecular testing (33), however, provides opportunity to determine the evolving role of mutation status and potential for minimally invasive treatment in these nodules.
Recurrent malignant lesions
While surgery is the preferred approach for recurrence of papillary thyroid cancer (PTC), patients may decline or be at high risk for complication due to co-morbidities or surgical history. RFA has been explored as an alternative therapy for these select patients, and shown promising results in patients with a limited number of small volume recurrences in the thyroid bed and/or lateral neck. In a study of 39 patients with 61 tumors ranging from 0.31 to 2.1 cm in greatest diameter, volume reduction after minimum 6 months of follow up was 95.1%. 82% of tumors completely disappeared on ultrasound, and average serum thyroglobulin decreased significantly (34). Recent long-term results have shown durable treatment response. Kim et al. reported a complete disappearance rate of 86.1% after 3 years in patients with local tumor recurrence under 2 cm in greatest diameter (35). In their study of 29 patients with 46 recurrent PTC lesions, Chung et al. reported mean volume reduction of 99.5% and complete disappearance of 91.3% of lesions after 80 months of follow up (36).
It should be noted that the rate of complications after RFA is higher in treating recurrent malignancy (10.98%) than benign nodules (1.27%) (37). This is not surprising given the anatomic alterations that result from prior surgical intervention, as well as the location of many recurrent lesions. Voice change, in particular, has been reported in nearly 8% of patients undergoing treatment for recurrent thyroid cancer (37). Strategies to mitigate voice change have been proposed, including instillation of cold dextrose in the tracheoesophageal groove until the voice has normalized (38). Relative to revision surgery, the risk profile remains favorable. In propensity score matching of 125 patients undergoing repeat surgery vs. 96 receiving RFA for recurrent thyroid cancer, Choi et al. reported similar rates of voice change but significantly higher incidence of hypoparathyroidism in the surgery group. Locoregional recurrence in the two groups was not significantly different (39).
Papillary microcarcinoma
There is increasing literature available regarding the efficacy and safety of RFA in treating papillary thyroid microcarcinoma (PTMC). Historically, treatment options for these typically indolent tumors have included surgery, which may involve a partial or total thyroidectomy, and active surveillance, which has been shown to have equivalent oncologic outcomes (40,41). However, tension exists between the discomfort of living with a known cancer, and wanting to avoid the risks of surgery. RFA may occupy the space between these approaches, offering a potentially therapeutic option with an improved safety profile relative to surgery.
In a large retrospective study of 414 patients with PTMC, mean volume reduction at 42 months was 98.81% with 88.41% rate of complete disappearance at last follow up (42). Overall tumor progression rate was 3.62%, with 10 patients developing recurrent PTMC, 4 showing cervical metastases, and 1 with residual PTMC. All patients with disease progression subsequently underwent additional successful RFA to address these lesions (42). Subsequent analyses have shown similarly promising results over long term follow up. In their study of 84 tumors in 74 patients, Cho et al. reported 100% complete disappearance rate at 60 months (43). Volume reduction of near 100% was achieved at two years, and maintained through the conclusion of the study. No patient developed locoregional or distant metastases or underwent delayed surgery. Four new foci of PTMC were detected and successfully treated with repeat RFA (43).
In comparison with surgery, RFA has been demonstrated to incur fewer complications and result in greater post-procedure quality of life, while maintaining similar oncologic control. Propensity-matched cohort study of 884 patients with PTMC treated with RFA or lobectomy and followed for four years demonstrated no significant difference in local tumor progression or recurrence free survival between the two groups (44). No complications were observed in the RFA group, while 4.5% of patients in the surgical group experienced temporary recurrent laryngeal nerve injury or hypocalcemia. Similarly, Zhang et al. reported no difference in disease progression in patients undergoing surgery vs. RFA at 64 months, but higher rate of complications (3.8% vs. 0%) in the surgery group (45). Importantly, quality of life at final follow up as measured by THYCA-QoL was significantly worse among surgical patients, which has been confirmed in a number of subsequent studies (45-47). While the precise role of and candidacy for RFA in PTMC has yet to be clearly defined, mounting evidence suggests RFA may have a legitimate place in the treatment paradigm of these small tumors with indolent courses.
Conclusions
Growing recognition of the importance of a precision approach to thyroid nodules has led to the international adoption of RFA and other ablative technologies. These have expanded the previously narrow space between surgical intervention and observation, providing a safe and less invasive therapeutic alternative. With conscientious patient selection and careful technique, RFA may be effectively applied to a variety of benign and malignant processes of the thyroid gland. The accumulation and analysis of efficacy and safety data from larger and more long-term prospective trials will more definitively establish its role relative to surgery and surveillance in each of these contexts.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ralph P. Tufano and Salem I. Noureldine) for the series “Novel Technology and Techniques in the Management of Thyroid Nodules” published in Annals of Thyroid. The article has undergone external peer review.
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-22-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-22-1/coif). The series “Novel Technology and Techniques in the Management of Thyroid Nodules” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008;22:901-11. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Schumm MA, Lechner MG, Shu ML, et al. Frequency of Thyroid Hormone Replacement After Lobectomy for Differentiated Thyroid Cancer. Endocr Pract 2021;27:691-7. [Crossref] [PubMed]
- Meyer C, Anderson D, Dong Z, et al. Prediction of Thyroid Hormone Replacement Following Thyroid Lobectomy: A Long-term Retrospective Study. OTO Open 2021;5:2473974X21992001.
- Kuo JH, McManus C, Lee JA. Analyzing the adoption of radiofrequency ablation of thyroid nodules using the diffusion of innovations theory: understanding where we are in the United States? Ultrasonography 2022;41:25-33. [Crossref] [PubMed]
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 2001;13:129-47. [Crossref] [PubMed]
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 2011;12:525-40. [Crossref] [PubMed]
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol 2019;180:79-87. [Crossref] [PubMed]
- Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European Thyroid Association Clinical Practice Guideline for the Use of Image-Guided Ablation in Benign Thyroid Nodules. Eur Thyroid J 2020;9:172-85. [Crossref] [PubMed]
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol 2018;19:632-55. [Crossref] [PubMed]
- Orloff LA, Noel JE, Stack BC Jr, et al. Radiofrequency ablation and related ultrasound-guided ablation technologies for treatment of benign and malignant thyroid disease: An international multidisciplinary consensus statement of the American Head and Neck Society Endocrine Surgery Section with the Asia Pacific Society of Thyroid Surgery, Associazione Medici Endocrinologi, British Association of Endocrine and Thyroid Surgeons, European Thyroid Association, Italian Society of Endocrine Surgery Units, Korean Society of Thyroid Radiology, Latin American Thyroid Society, and Thyroid Nodules Therapies Association. Head Neck 2022;44:633-60. [Crossref] [PubMed]
- Vuong NL, Dinh LQ, Bang HT, et al. Radiofrequency Ablation for Benign Thyroid Nodules: 1-Year Follow-Up in 184 Patients. World J Surg 2019;43:2447-53. [Crossref] [PubMed]
- Rabuffi P, Spada A, Bosco D, et al. Treatment of thyroid nodules with radiofrequency: a 1-year follow-up experience. J Ultrasound 2019;22:193-9. [Crossref] [PubMed]
- Trimboli P, Bini F, Marinozzi F, et al. High-intensity focused ultrasound (HIFU) therapy for benign thyroid nodules without anesthesia or sedation. Endocrine 2018;61:210-5. [Crossref] [PubMed]
- Lim HK, Lee JH, Ha EJ, et al. Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 2013;23:1044-9. [Crossref] [PubMed]
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia 2017;33:905-10. [Crossref] [PubMed]
- Jung SL, Baek JH, Lee JH, et al. Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study. Korean J Radiol 2018;19:167-74. [Crossref] [PubMed]
- Deandrea M, Trimboli P, Garino F, et al. Long-Term Efficacy of a Single Session of RFA for Benign Thyroid Nodules: A Longitudinal 5-Year Observational Study. J Clin Endocrinol Metab 2019;104:3751-6. [Crossref] [PubMed]
- Yan L, Zhang M, Xie F, et al. Efficacy and safety of radiofrequency ablation for benign thyroid nodules in patients with previous thyroid lobectomy. BMC Med Imaging 2021;21:47. [Crossref] [PubMed]
- Ji Hong M, Baek JH, Choi YJ, et al. Radiofrequency ablation is a thyroid function-preserving treatment for patients with bilateral benign thyroid nodules. J Vasc Interv Radiol 2015;26:55-61. [Crossref] [PubMed]
- Che Y, Jin S, Shi C, et al. Treatment of Benign Thyroid Nodules: Comparison of Surgery with Radiofrequency Ablation. AJNR Am J Neuroradiol 2015;36:1321-5. [Crossref] [PubMed]
- Monpeyssen H, Alamri A, Ben Hamou A. Long-Term Results of Ultrasound-Guided Radiofrequency Ablation of Benign Thyroid Nodules: State of the Art and Future Perspectives-A Systematic Review. Front Endocrinol (Lausanne) 2021;12:622996. [Crossref] [PubMed]
- Bernardi S, Dobrinja C, Fabris B, et al. Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014;2014:934595. [Crossref] [PubMed]
- Wang N, Zheng B, Wu T, et al. Thyroid dysfunction following radiofrequency ablation for benign thyroid nodules: more likely to occur within one-week and in high-risk population. Int J Hyperthermia 2021;38:1060-8. [Crossref] [PubMed]
- Chung SR, Baek JH, Sung JY, et al. Revisiting Rupture of Benign Thyroid Nodules after Radiofrequency Ablation: Various Types and Imaging Features. Endocrinol Metab (Seoul) 2019;34:415-21. [Crossref] [PubMed]
- Bernardi S, Stacul F, Michelli A, et al. 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine 2017;57:402-8. [Crossref] [PubMed]
- Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency Ablation on Autonomously Functioning Thyroid Nodules: A Critical Appraisal and Review of the Literature. Front Endocrinol (Lausanne) 2020;11:317. [Crossref] [PubMed]
- Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperthermia 2019;36:376-82. [Crossref] [PubMed]
- Mader A, Mader OM, Gröner D, et al. Minimally invasive local ablative therapies in combination with radioiodine therapy in benign thyroid disease: preparation, feasibility and efficiency - preliminary results. Int J Hyperthermia 2017;33:895-904. [Crossref] [PubMed]
- Ha SM, Sung JY, Baek JH, et al. Radiofrequency ablation of small follicular neoplasms: initial clinical outcomes. Int J Hyperthermia 2017;33:931-7. [Crossref] [PubMed]
- Dobrinja C, Bernardi S, Fabris B, et al. Surgical and Pathological Changes after Radiofrequency Ablation of Thyroid Nodules. Int J Endocrinol 2015;2015:576576. [Crossref] [PubMed]
- Lin WC, Tung YC, Chang YH, et al. Radiofrequency ablation for treatment of thyroid follicular neoplasm with low SUV in PET/CT study. Int J Hyperthermia 2021;38:963-9. [Crossref] [PubMed]
- Mitchell J, Yip L. Decision Making in Indeterminate Thyroid Nodules and the Role of Molecular Testing. Surg Clin North Am 2019;99:587-98. [Crossref] [PubMed]
- Lim HK, Baek JH, Lee JH, et al. Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol 2015;25:163-70. [Crossref] [PubMed]
- Kim JH, Yoo WS, Park YJ, et al. Efficacy and Safety of Radiofrequency Ablation for Treatment of Locally Recurrent Thyroid Cancers Smaller than 2 cm. Radiology 2015;276:909-18. [Crossref] [PubMed]
- Chung SR, Baek JH, Choi YJ, et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol 2019;29:4897-903. [Crossref] [PubMed]
- Chung SR, Suh CH, Baek JH, et al. Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia 2017;33:920-30. [PubMed]
- Lee MK, Baek JH, Chung SR, et al. Effectiveness of Injecting Cold 5% Dextrose into Patients with Nerve Damage Symptoms during Thyroid Radiofrequency Ablation. Endocrinol Metab (Seoul) 2020;35:407-15. [Crossref] [PubMed]
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia 2019;36:359-67. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, et al. Incidences of Unfavorable Events in the Management of Low-Risk Papillary Microcarcinoma of the Thyroid by Active Surveillance Versus Immediate Surgery. Thyroid 2016;26:150-5. [Crossref] [PubMed]
- Cho SJ, Suh CH, Baek JH, et al. Active Surveillance for Small Papillary Thyroid Cancer: A Systematic Review and Meta-Analysis. Thyroid 2019;29:1399-408. [Crossref] [PubMed]
- Yan L, Lan Y, Xiao J, et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol 2021;31:685-94. [Crossref] [PubMed]
- Cho SJ, Baek SM, Lim HK, et al. Long-Term Follow-Up Results of Ultrasound-Guided Radiofrequency Ablation for Low-Risk Papillary Thyroid Microcarcinoma: More Than 5-Year Follow-Up for 84 Tumors. Thyroid 2020;30:1745-51. [Crossref] [PubMed]
- Yan L, Zhang M, Song Q, et al. Ultrasound-Guided Radiofrequency Ablation Versus Thyroid Lobectomy for Low-Risk Papillary Thyroid Microcarcinoma: A Propensity-Matched Cohort Study of 884 Patients. Thyroid 2021;31:1662-72. [Crossref] [PubMed]
- Zhang M, Tufano RP, Russell JO, et al. Ultrasound-Guided Radiofrequency Ablation Versus Surgery for Low-Risk Papillary Thyroid Microcarcinoma: Results of Over 5 Years' Follow-Up. Thyroid 2020;30:408-17. [Crossref] [PubMed]
- Lan Y, Zhang MB, Zhang Y, et al. Comparison of Quality of Life of Patients with Papillary Thyroid Microcarcinoma Treated by Different Modalities. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2021;43:328-37. [PubMed]
- Lan Y, Luo Y, Zhang M, et al. Quality of Life in Papillary Thyroid Microcarcinoma Patients Undergoing Radiofrequency Ablation or Surgery: A Comparative Study. Front Endocrinol (Lausanne) 2020;11:249. [Crossref] [PubMed]
Cite this article as: Noel JE, Hannabass K, Orloff LA. Radiofrequency ablation of benign and malignant thyroid nodules: updates and current status. Ann Thyroid 2022;7:7.


