
Case report: poorly differentiated thyroid carcinoma in a young female patient
Introduction
Poorly differentiated thyroid carcinoma (PDTC) is a rare form of thyroid cancer that represents 4–7% of all thyroid carcinomas and, rarer still, is the occurrence in those under the age of 21 (1). The etiology of the disease is largely unknown but thought to arise from well differentiated thyroid carcinoma (WDTC) with the potential to advance to anaplastic thyroid carcinoma (ATC). This case report seeks to present and discuss PDTC in the form of patient presentation, workup, microscopic findings, the Turin criteria, and genetic analysis to further the understanding of PDTC, specifically in patients <21. It will largely focus on genetic findings which include, a lack of a DICER1 mutation, a MYC amplification, and a variety of mutations with unknown clinical significance. We present the following case in accordance with the CARE reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-21-20/rc).
Case presentation
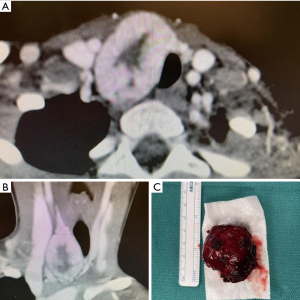
A young previously healthy female (<21) patient presented with a large firm neck mass right of midline and adjacent to the thyroid gland without other signs or symptoms. Family history was negative for pertinent findings. A CT scan showed the mass appeared potentially contiguous with the right thyroid lobe with mild tracheal compression (Figure 1). Fine needle aspiration and cytology reported mixed solid, acinar, and focal microfollicular architecture. It was thus signed out as being most consistent with a benign adenomatous nodule with recommended correlation with imaging. The mass was resected with a right hemithyroidectomy and sent for further histopathological evaluation which included frozen sections, standard H&E staining, and immunohistochemistry. The patient was subsequently referred to a tertiary cancer treatment center and follow up information was unable to be obtained. Despite multiple attempts, informed consent was unable to be obtained from the patient as follow-up was lost. The patient’s contact information was unreliable at the time this case report was written. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013).
Timeline of events
Late 2020:
- Patient presents complaining of a solid neck mass, confirmed with palpation and visual inspection by PCP. Referred for ultrasound evaluation.
- Ultrasound identifies a homogenous hypoechoic neck mass with prominent vasculature, without definitive involvement of the right thyroid lobe. FNA is ordered.
- FNA and subsequent cytopathologic findings give the preliminary diagnosis favoring a benign follicular lesion. Patient is referred to a surgeon for mass resection.
Early 2021:
- Surgeon orders a CT scan which confirms size and location of the mass in addition to mild tracheal compression. The patient remained asymptomatic during this time.
- A right hemithyroidectomy is performed and the mass is removed without complications.
- Initial frozen sections were deferred to permanent due to the complexity of the lesion.
- Permanent sections were then sent from the community hospital setting to a university hospital. An expert on thyroid pathology is also consulted during this time.
- The three entities agree on the final diagnosis of PDTC, likely arising from a prior papillary thyroid carcinoma (PTC), follicular type.
- Genetic analysis is recommended due to the rarity of this tumor in this age group, looking for a DICER1 mutation.
- Patient is also referred to a tertiary cancer treatment center and follow-up is lost.
Mid 2021:
- Genetic testing is completed and no DICER1 mutation was found. Instead, a MYC amplification and multiple other mutations are identified. These findings are reported out and an addendum to the case is made.
Macroscopic description
A 22 g red 4.0×3.5×2.5 cm3 nodule. Sectioning the specimen showed a fleshy, red-tan cut surface.
Microscopic description
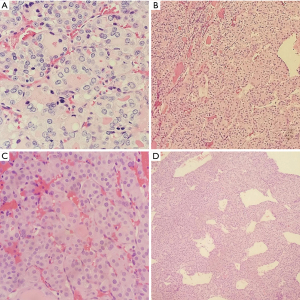
Frozen section slides showed a follicular lesion, but definitive diagnosis was deferred to permanents. Permanent histological examination reveals an insular, solid, and trabecular growth pattern (Figure 2) comprised of small, poorly formed follicles with loss of PTC nuclear features in a vast majority of lesions with several lesser areas showing PTC-like nuclear features. Within the trabecular and solid architecture there are multiple foci of necrosis and dispersed mitotic figures. There is a background of normal looking thyroid follicles scattered sparsely throughout with colloid remnants. Notable is the tumor’s invasion of both the lymphovasculature and nervous tissue in the periphery (Figure 3). Although partially encapsulated, the tumor is freely infiltrative. Individual cells have normal to enlarged nuclei, prominent nucleoli, and an abundance of eosinophilic cytoplasm. The nuclei are relatively round with and are lacking nuclear grooves, Pseudonuclear inclusion bodies, and nuclear clearing (Orphan-Annie eye).
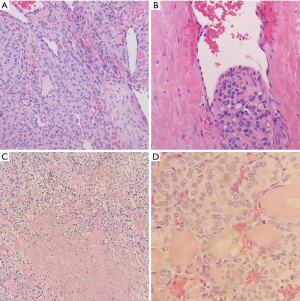
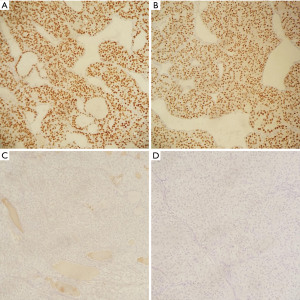
Immunohistochemistry staining showed TTF1 positive follicular cells in the lesion, helping rule out ATC. PAX8 was also positive confirming the lesion is of thyroid origin. Staining was negative for synaptophysin and chromogranin (Figure 4). A Congo red stain was performed, and polarization was negative for amyloid.
Diagnosis and therapeutic intervention
The frozen section diagnosis given was: Follicular lesion, defer to permanents. The final diagnosis was PDTC likely arising from a PTC, follicular variant.
An extensive workup was used to diagnose the mass including ultrasound, a core needle biopsy, CT scan with contrast, and ultimately surgical excision followed by multiple pathology consults. The patient continued to be asymptomatic and recovered well from the right hemithyroidectomy. No extrathyroidal extension was identified clinically. It was recommended the patient follow-up to completely resect the remaining thyroid and seek genetic testing for a DICER1 mutation. Neoadjuvant chemotherapy is also commonly used to treat the disease, although it is unclear if this patient pursued this option.
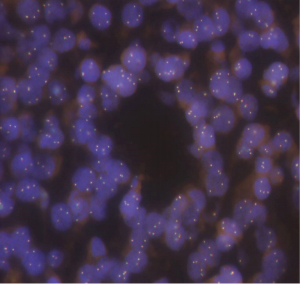
Genetic testing on the formalin fixed paraffin embedded tissue (NeoTYPE Discovery Profile for Solid Tumors from Neogenomics Laboratories) was negative for suspected DICER1 mutation that can be seen in young patients with PDTC that carries an elevated risk of developing additional cancers (2). The panel used tests for well over 100 biomarkers and resulted in pertinent negatives such as BRAF, BRCA1, BRCA2, EGFR, KIT, KRAS, NRAS, PDGFRA, PIK3CA, ALK FISH, MET FISH, PDGFRe FISH, PTEN FISH, RET FISH, and ROS1 FISH. Other pertinent findings from the genetic analysis included a MYC amplification by fluorescent in situ hybridization (FISH) with 4 or more MYC signal per nucleus in at least 40% of tumor cells (48%) consistent with gene amplification (Figure 5). Additional copies of BRAF (>2F, 80.0%, negative <25.1%) [Fusion cutoff, % of cells counted, Negative cutoff], RET (>2F, 24.0%, negative <16.2%), and PTEN (>2R>2G, 32.0%, negative <21.8%) were observed which is an abnormal result suggestive of gains of BRAF (7q34) or chromosome 7, RET (10q11), PTEN (10q23) or chromosome 10. No additional known mutations of clinical significance were identified; although 5 variants of unknown clinical significance were present in the BLM (D88E NM_000057.4:c.264C>A), KMT2A (E495K NM_005933.4:c.1483G>A), MPL (T469I NM_005373.3:c.1406C>T), NF2 (R338C NM_000268.3:c.1012C>T) and PRKDC (A2345V NM_006904.7:c.7034C>T) gene loci. Prognosis in this case of a young patient without a DICER1 mutation is still largely unknown (3).
Discussion
PDTC is a relatively rare and aggressive form of thyroid cancer making up approximately 4–7% of all thyroid carcinomas. PDTC occurs most commonly in older populations with the mean age of clinical detection being 57 years with a predominately female predilection (2). It typically presents as a painless thyroid mass averaging 4.0 cm in diameter at initial presentation and may affect one or both thyroid lobes. It may arise from a precursor lesion or without a prior lesion. Precursor lesions include WDTC such as follicular or PTC as in this case. The diagnosis can often be missed, lending itself to being the highest cause of fatalities from non-anaplastic thyroid entities if not considered in the differential (4-6). PDTC looks and behaves as an intermediate between WDTC and ATCs. Extrathyroidal, regional, and distant spread are common, with studies showing 70%, >50%, and 43% respectively (7). The etiology of this tumor is poorly understood.
The Turin criteria (3 features) are used for definitive diagnosis of PDTC. The growth pattern is shown to be trabecular, solid, or insular (Turin criteria 1). There must be no nuclear features of PTC (Turin criteria 2) and the tumor must have at least one of the three following features: convoluted nuclei, ≥3 mitotic figures per 10 high power fields (HPF), or necrosis (Turin criteria 3) (7). These criteria are necessary due to the ambiguous histopathologic features of the tumor as it can have focal areas of WDTC amongst prominent areas of atypia that do not reach the levels seen in ATC.
Given the rarity of this tumor type in younger age groups, it was suspected to be associated with DICER syndrome caused by a mutation in the DICER1 gene. The DICER1 gene is responsible for encoding the DICER endoribonuclease protein that is important in the control of protein translation, specifically microRNAs (miRNAs). As a component of the RNA induced silencing complex (RISC), DICER’s role is to transform the single stranded pre-miRNAs into double stranded RNAs that can then be transported and bind to specific mRNAs. The binding of the miRNA to the mRNA prevents the attachment of ribosomes and subsequently, any protein translation that would have followed (2,8). It is thought that the absence of a functional DICER protein may contribute to either a loss of function in tumor suppressor genes, or a gain-of-function in oncogenic genes that are regulated by the presence of inhibitory miRNA. Defective, downregulated, or even over expression of miRNAs resulting from a DICER1 mutation may lead to the development of cancer, or a poor prognosis in cancer (9-11).
DICER syndrome is a rare disorder characterized by a predisposition to multiple cancers including but not limited to Sertoli-Leydig tumors, pleuropulmonary blastoma, multinodular goiter, cystic nephromas, and PDTC (2). One study showed that PDTC in young patients is likely due to a distinct etiology associated with DICER1, although its sample size was extremely limited. 5 out of 6 patients <21 years with PDTC were found to have DICER1 mutations and no additional known mutations associated with other thyroid neoplasms. Three out of the five patients died within 8–24 months due to their disease (3). In this case, it is recommended to do a genetic evaluation in order to better assess the patient’s potential for developing additional malignancies and overall prognosis.
A notable finding in this case was the presence of a MYC amplification using FISH. MYC is a well-known proto-oncogene that shows overexpression in approximately 20% of all human cancers and is a poor prognostic indicator (12,13). Chernock et al. (3) found that 1 of 6 patients <21 years diagnosed with PDTC did not have a DICER1 mutation, but it was not mentioned if there were any additional mutations or other genetic abnormalities that may be implicated in oncogenesis. Current studies have not shown a direct connection between MYC and PDTCs, but it can be assumed as MYC amplifications have been found in PTCs and may encourage dedifferentiation. These findings support prior evidence that PDTC can be a progression from WDTC (14). The MYC amplification’s significance in this case can be implied by its frequent association with other cancers, but definitive significance is uncertain. Unfortunately, since follow-up with the patient was lost, it cannot be determined what the clinical outcome of the treated disease was, as well as the implications of the genetic findings. As research on PDTC continues, the etiology of the disease may become clearer, especially in its poorly understood occurrence in younger populations. Currently our understanding of PDTC etiology in children is limited, and additional studies focused on genetic markers may prove useful.
Notably, a recently published case of a metastatic PDTC arising from a PTC in a 28-year-old patient had shown a germline MET mutation (15). The MET gene encodes a receptor tyrosine kinase, which in tandem with the MET ligand, hepatocyte growth factor (HGF), stimulates the MET-HGF pathway resulting in cellular proliferation, mitosis, apoptosis and motility (15,16). Mutations in the well-known MET oncogene can also be found in a variety of cancers not unlike MYC gene amplifications and may play a role in their dedifferentiation. Our case was negative for any MET abnormalities.
Additional findings of undetermined clinical significance in the genetic analysis of this patient include mutations in the KMT2A, NF2, BLM, MPL, and PRKDC genes. KMT2A is an epigenetic regulator and is one of the most frequently mutated histone methyltransferases. Mutations of this gene are considered clinically insignificant, but its mutations have been implicated in the pathogenesis of thyroid cancer (17). According to AACR project Genie, NF2 mutations are common in ATC (8.06%) and not in PDTC (0.64%). This is for patients with ATC that have NF2 mutations (in the tumor) without NF2 disease (18). BLM and MPL mutations have also been identified in thyroid cancer, although their correlation is unknown. BLM encodes for helicase enzymes and MPL encodes for thrombopoietin receptor protein (19). PRKDC is a DNA double stranded break repair enzyme, and its amplification has been implicated in a variety of cancers. There is no documented correlation with the development of thyroid cancer. These variant mutations in addition to MYC amplification may support the overall picture of PDTC arising from a previous WDTC through acquired mutations. Research by Landa et al. shows evidence of cumulative mutations as a major factor in the development of PDTC or ATC that may be of clinical value (20).
This case provides a histopathological overview of PDTC as well as relevant insight into the possible etiology of this poorly understood disease. Although one small study has shown a connection between PDTC and DICER1 syndrome, this case did not, and instead describes another means for young patients to develop this tumor type. We hope this case report is useful in further defining genetic risk factors and assists in identification of PDTC, especially in young patients.
Practice points
PDTC is a rare thyroid cancer with its own distinct criteria for diagnosis. It mainly presents in older adults but can occur in children and adolescents.
The etiology of PTDC can be associated with a genetic component such as DICER1 mutations, or in this case, MYC amplification. It can arise from a preexisting well-differentiated thyroid carcinoma or de novo.
DICER1 mutation should be strongly suspected when a PTDC is diagnosed in children or adolescents, although more research is needed as this case shows that it is not always present.
The Turin criteria help a great deal in diagnosing this type of cancer due to its ambiguity.
Self-assessment questions
- Which IHC stain’s positivity can help differentiate between PDTC and ATC while maintaining its specificity for a tumor of thyroid origin?
- PAX8
- TTF1
- Chromogranin
- Synaptophysin
- Calcitonin
- Per the TURIN guidelines, which of the following is one of the diagnostic criteria for PDTC?
- Lymphovascular invasion
- Distant metastasis
- Presence of nuclear features such as nuclear clearing, nuclear grooves, and pseudonuclear inclusion bodies.
- >3 mitotic figures/10 HPF.
- Arising from a background of a WDTC.
Answers
- B—TTF1 (ATC almost always loses its TTF1 staining but retains PAX8).
- D—>3 mitotic figures/10 HPF (one of the 3 sub-criteria with the other two being necrosis and convoluted nuclei. These can be present either individually or together).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-21-20/rc
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-21-20/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-21-20/coif). PA and JM do disclose that they are employed by for-profit healthcare organizations. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Informed Consent was unable to be obtained from the patient as follow-up was lost. The patient’s contact information was unreliable at the time this case report was being written.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tanaka K, Sonoo H, Saito W, et al. Analysis of clinical outcome of patients with poorly differentiated thyroid carcinoma. ISRN Endocrinol 2011;2011:308029. [Crossref] [PubMed]
- Robertson JC, Jorcyk CL, Oxford JT. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers (Basel) 2018;10:143. [Crossref] [PubMed]
- Chernock RD, Rivera B, Borrelli N, et al. Poorly differentiated thyroid carcinoma of childhood and adolescence: a distinct entity characterized by DICER1 mutations. Mod Pathol 2020;33:1264-74. [Crossref] [PubMed]
- Dettmer MS, Schmitt A, Komminoth P, et al. Poorly differentiated thyroid carcinoma: An underdiagnosed entity. Pathologe 2020;41:1-8. [Crossref] [PubMed]
- Cherkaoui GS, Guensi A, Taleb S, et al. Poorly differentiated thyroid carcinoma: a retrospective clinicopathological study. Pan Afr Med J 2015;21:137. [Crossref] [PubMed]
- Ibrahimpasic T, Ghossein R, Shah JP, et al. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid 2019;29:311-21. [Crossref] [PubMed]
- Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 2007;31:1256-64. [Crossref] [PubMed]
- Murray MJ, Bailey S, Raby KL, et al. Serum levels of mature microRNAs in DICER1-mutated pleuropulmonary blastoma. Oncogenesis 2014;3:e87. [Crossref] [PubMed]
- Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol 2012;6:590-610. [Crossref] [PubMed]
- Chiosea S, Jelezcova E, Chandran U, et al. Up-regulation of dicer, a component of the MicroRNA machinery, in prostate adenocarcinoma. Am J Pathol 2006;169:1812-20. [Crossref] [PubMed]
- Zhu DX, Fan L, Lu RN, et al. Downregulated Dicer expression predicts poor prognosis in chronic lymphocytic leukemia. Cancer Sci 2012;103:875-81. [Crossref] [PubMed]
- McAnulty J, DiFeo A. The Molecular 'Myc-anisms' Behind Myc-Driven Tumorigenesis and the Relevant Myc-Directed Therapeutics. Int J Mol Sci 2020;21:9486. [Crossref] [PubMed]
- Schaub FX, Dhankani V, Berger AC, et al. Pan-cancer Alterations of the MYC Oncogene and Its Proximal Network across the Cancer Genome Atlas. Cell Syst 2018;6:282-300.e2. [Crossref] [PubMed]
- Zhang Y, Li F, Chen J. MYC promotes the development of papillary thyroid carcinoma by inhibiting the expression of lncRNA PAX8-AS1:28. Oncol Rep 2019;41:2511-7. [Crossref] [PubMed]
- Johansson K, Stenman A, Paulsson JO, et al. Development of metastatic poorly differentiated thyroid cancer from a sub-centimeter papillary thyroid carcinoma in a young patient with a germline MET mutation - association or random chance? Thyroid Res 2021;14:19. [Crossref] [PubMed]
- Cecchi F, Rabe DC, Bottaro DP. The Hepatocyte Growth Factor Receptor: Structure, Function and Pharmacological Targeting in Cancer. Curr Signal Transduct Ther 2011;6:146-51. [Crossref] [PubMed]
- Swierniak M, Pfeifer A, Stokowy T, et al. Somatic mutation profiling of follicular thyroid cancer by next generation sequencing. Mol Cell Endocrinol 2016;433:130-7. [Crossref] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov 2017;7:818-31. [Crossref] [PubMed]
- Pitt SC, Hernandez RA, Nehs MA, et al. Identification of Novel Oncogenic Mutations in Thyroid Cancer. J Am Coll Surg 2016;222:1036-1043.e2. [Crossref] [PubMed]
- Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016;126:1052-66. [Crossref] [PubMed]
Cite this article as: Kaysen S, Anderson P, Mohlman J. Case report: poorly differentiated thyroid carcinoma in a young female patient. Ann Thyroid 2022;7:5.


