
Narrative review: iodine—thyroidal and extrathyroidal actions
Introduction
The best recognized role for iodine in mammalian biology is as a constituent of thyroid hormones (1). However, iodine has additional little recognized roles in other organs, notably the breast, placenta, stomach and intestines, kidneys, parotid, and skin. Iodine in breast milk plays an important role in neonatal nutrition and may be involved in maintaining breast physiology (2,3). Association between iodine, the thyroid and breast cancer has been the subject of many studies (4). The presence of iodine and its transport in a range of tissues has been described (5), although most of its extrathyroidal functions remain unknown. These may reflect its role as a potent antioxidant in humans (3) and its presence in seawater represents an important natural antioxidant (6). In humans the total amount of iodine is about 30 mg with approximately 15–20 mg in the thyroid, the remainder distributed throughout the body (2,7). Apart from some coastal areas, iodine is usually present at low concentrations in soils. Higher iodine concentrations present in mineral deposits, in underground brines accessed during oil exploration and in Chilean caliche ore form the major commercial source (8). Fish, crustaceans of marine origin and seaweeds provide the most abundant food sources of iodine. However, in many countries iodised table salt is the main dietary source (9,10). Iodine is either directly absorbed into the bloodstream as iodide (I−) or converted to (I−) in the gut, absorbed into the bloodstream or excreted in the urine. In an iodine replete individual about 95% of daily intake is excreted in the urine with absorption depending on chemical form and bioavailability (11,12). Urinary iodine (UI) estimation provides an accurate index of iodine intake, but its assessment can be problematic (12). Variation in daily iodine intake means that UI is unsuited for determining individual iodine status. Iodine intake is mainly through oral consumption although other routes such as through the skin or via respiration have been described (13,14). Whatever the mode of uptake, absorption of iodine into the cell depends on the sodium iodide symporter (NIS) (15) which is found predominantly on the cell membrane of the thyroid follicular cell but is also significantly expressed in other tissues such as the lactating breast, parotid and placenta (16-18). While iodine intake is necessary for normal physiology, higher amounts by downregulating NIS may result in inhibition of thyroid hormone formation and release (19). In addition to its role in human biology, gaseous iodine (I2) in the marine environment plays an important role, particularly in coastal areas, in influencing weather patterns through cloud formation (20). I present the following article in accordance with the Narrative Review reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-21-28/rc).
Methods
The search strategy summary is shown in Table 1.
Table 1
| Terms | Specification |
|---|---|
| Date of search (specified to date, month and year) | March 2019 |
| Databases and other sources searched | PubMed, Google |
| Search terms used (including MeSH and free text search terms and filters) | Iodine, iodine deficiency, iodine excess, iodine in extrathyroidal disorders, atmospheric iodine, assessment of iodine |
| Timeframe | 1990–2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | English language |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Author |
| Any additional considerations, if applicable | NA |
NA, not applicable.
Iodine and the thyroid
The major function of iodine is in the formation of the thyroid hormones T4 and T3. The biological action of thyroid hormones depends on the deiodinating enzymes, deiodinase 1, 2 and 3 (D1, D2 and D3) present in liver, kidney, and in many peripheral tissues where they serve to maintain biologically active T3 concentrations (21). As iodine is not manufactured in the body it must be taken in from dietary or environmental sources. Iodine absorption is through the gut into the bloodstream from where it is carried to the thyroid and some extrathyroidal organs (15). Since most iodine intake is discretionary, an understanding and avoidance of the consequences of iodine deficiency or excess is required.
Iodine: sources and dietary supplementation
The marine environment provides the major source of iodine. Although the concentration of iodine in seawater is relatively low (approx. 58 µg/L), the great mass of the oceans makes it the major source of iodine on the planet. Iodine in sea water is mainly present as I− and IO3, with I− predominating in surface waters while IO3 forms the greatest proportion in deep water (22). Products of the sea such as seafish, crustaceans and seaweeds provide the richest sources of dietary iodine intake (9,23). However, in many countries milk and dairy products are predominant providers particularly where seafood, due to cost or availability, may not be favoured (9,10). The daily intake of iodine recommended by the WHO is shown in Table 2. Dietary intake of dairy products is not ubiquitous; therefore, iodisation of table salt with Ki or KIO3 at a level of 20–40 mg/kg. Universal salt iodisation (USI) provides a useful method of achieving population iodine cover (9,23). Use of iodised table salt is only mandatory in some countries and is almost entirely absent in others such as the UK and Ireland (23). Even in the absence of discretionary use of table salt, addition through seasoning during cooking and eating represents only approximately 40% of salt consumed, the balance being made up by salt containing processed foods (24). Another source of iodine supplementation is fortification of flour used in bread making. This approach is mandatory on both Australia and New Zealand (25,26). As a result of increased use of iodised salt, the number of countries classified as iodine deficient has declined over recent decades (27). Introduction of salt iodisation is frequently opposed on the basis of contributing to hypertension when in fact what is proposed is not increasing salt intake but altering the chemical composition of table salt to include KI or KIO3 (28). Despite its benefits for increasing iodine intake, iodised salt does not completely solve the problem of iodine deficiency. Many vulnerable groups where iodine requirement is increased such as pregnant mothers, their infants, vegans, or women of childbearing age continue to show deficits in iodine intake despite the availability of iodised salt (23,29). Even where USI is practiced such as in Switzerland, high risk groups such as pregnant women do not achieve iodine sufficiency. Achieving this goal appears to require the use of iodised salt in the production of processed foods (29). Some dietary supplements such as vitamin pills have added iodine at concentrations that do not exceed WHO recommendations (23). Similarly, seaweeds consumed as foodstuffs can provide adequate iodine but some seaweed products such as kelp tablets contain excessive iodine with dangers of iodine excess if habitually consumed (23).
Table 2
| Group | Daily iodine intake |
|---|---|
| Preschool children 0–59 months | 90 |
| Schoolchildren 6–12 years | 120 |
| Adolescents above 12 years | 150 |
| Adults | 150 |
| Pregnant and lactating women | 250 |
Iodine deficiency: endemic goitre
Although severe iodine deficiency is thankfully no longer a major worldwide problem, inadequate iodine intake is still frequently encountered and maternal iodine deficits may lead to developmental or neurological problems, particularly in the fetus and neonate (9). Such deficits may also affect those consuming vegetarian or vegan diets (30). Severe iodine deficiency may, through decreased formation of thyroid hormones, result in endemic cretinism which can present either as myxedematous or neurological cretinism (31-33). The former is characterised by neonatal hypothyroidism and irreversible mental retardation. The neurological cretin also has neurological disorders but is not hypothyroid. The difference in presentation of the two forms of cretinism has been attributed to low dietary selenium (Se) leading to decreased selenoenzyme D1 and diminished T4 to T3 conversion (31). Se intake varies across the world owing largely to differences in soil content and factors affecting its bioavailability to plants (32). Se and iodine share a common provenance in that neither is manufactured in the body and both require dietary input. Adequate intakes of both iodine and selenium are required for optimal thyroid function as Se forms an important part of selenoenzymes including deiodinases necessary for thyroid hormone action (31). Another selenoenzyme is glutathione thyroperoxidase an important agent for removing potentially harmful reactive oxygen species including H2O2 involved in the production of thyroid hormones (31,32).
When Se and iodine deficiency are combined, low thyroid hormone levels will increase TSH driving H2O2 formation and accumulation which is cytotoxic to thyroid cells resulting in the hypothyroidism seen in myxedematous cretinism (31-33). The importance of simultaneously replenishing both iodine and Se when treating endemic goitre was shown by the decrease in serum T4 and worsening of endemic hypothyroidism brought about when only Se was administered (33).
Iodine deficiency in pregnancy and the neonate
Iodine deficiency of a more moderate or borderline nature remains a worldwide problem afflicting both underdeveloped and developed nations (9). The major consequence of iodine deficiencies applies in the first weeks of life, as a functioning thyroid gland is not evident until about 18–20 weeks gestation during which time the fetus is totally dependent on the mother producing thyroid hormones necessary for growth and neuropsychological development (17). Thus, maternal iodine intake is necessary for both mother and baby to thrive and even mild iodine deficiency can lead to lower cognitive development (34). This increased requirement prompted the WHO to recommend that the adult daily iodine requirement of 150 µg be increased to 250 µg in pregnant and lactating women (see Table 2). However, a recent report from China showed that thyroid disease rates in previously iodine deficient areas were lower in pregnant women with UIC 100–149 µg/L. The authors suggested that the optimal UIC criteria recommended by WHO for pregnant women may be a little excessive particularly where the preceding iodine status ranged from mild-to-moderate iodine-deficient (35). Although the consequences of severe iodine deficiency in pregnancy have been well documented (9,10), diminished iodine intake can result in hypothyroxinemia and inadequate thyroid hormone supply to the developing fetal brain, the consequences of which can be orders of magnitude higher than those observed in congenital hypothyroidism (CHO) (36). Isolated hypothyroxinemia rates of approximately 1.3% have been reported (37) which contrasts to a CHO rate of approximately 0.04% (38).
Iodine: high dietary intake
A high dietary iodine intake is frequently attributed to consumption of marine products, particularly seaweeds (23). Brown seaweeds (kelps) are generally more iodine rich than the green or red varieties. The use of seaweed in Asian cooking has been long established and is enjoying-increasing popularity in the Western diet (39). Consuming excess iodine can result in thyroid function disorders including goitre, thyroid autoimmunity, hypothyroidism or hyperthyroidism (40,41). There continues to be a problem in defining what constitutes iodine excess with a tolerable upper limit (TUL) varying from 600 to 900–1,100 µg (42,43). As over 90% of daily injected iodine is excreted in the urine, it is probable that any antithyroid effect is dependent on prolonged ingestion, the existing thyroid iodine content, and the state of health of thyroid tissue. A decrease in goitre and hyperthyroidism, mainly toxic nodular goitre, has been shown in Denmark following iodisation of table salt but a small increase in hypothyroidism was noted (44). The authors noted that even small systematic increase in iodine supply can significantly increase the risk of thyroid disease and emphasised the importance of increasing iodine intake to the level where iodine deficiency disorders (IDD) are prevented and not higher (45). However, a report from China suggested that hyperthyroidism prevalence remained stable two decades after USI (46). An anomaly in determining what is safe dietary iodine intake is provided by the Japanese experience. Iodine rich seaweed is consumed in approximately 21% of Japanese meals yielding an average iodine intake of 1.2 mg/day with some subjects consuming considerably more (47,48). It appears that Japanese thyroids have adapted to a high iodine intake without an increased rate of thyroid disorders. In contrast to the Danish experience there was no sudden increase in iodine intake. An interesting exception was provided by the finding of iodide induced goitre first reported in the residents of the island of Hokkaido, Japan termed “Endemic Coastal Goitre” where iodine consumption averaged 20 mg, daily which was excessive even by Japanese standards (48). A possible explanation of thyroidal adaption to high iodine loads was suggested to result from an iodostat set according to the habitual dietary iodine intake of the population (49).
A high iodine intake in patients with autonomously functioning nodules perhaps arising from a previous iodine deficiency state can result in a form of hyperthyroidism termed Jod Basedow (50,51). Amiodarone induced thyrotoxicosis (AIT 1) or AIT 2 characterised by destructive thyroiditis, can result from treatment with the cardiac antiarrhythmic drug amiodarone (contains 75 mg iodine per tablet) (50). Similarly, contrast media used in radiologic examinations or iodine rich disinfectants liberally applied which in susceptible individuals can result in thyroid dysfunction, frequently hypothyroidism (51). Other sources of excess iodine intake include dietary supplements such as kelp tablets, food colourings such as erythrosine and occasionally cough mixtures, although many of these are now iodine free (41). High iodine levels can diminish the effectiveness of RAI therapy for hyperthyroidism or thyroid cancer with dietary iodine restriction often advocated (19). Not all excess iodine intake has damaging consequences. High iodine content (approx. 100 mg) KI or KIO3 tablets is often administered as a protective blocking agent to populations at risk of ingesting RAI arising from a nuclear accident. It can be speculated that the consequences of RAI uptake and future thyroid cancers following the 2011 Fukushima accident would have been much worse in a population other than seaweed consuming iodine replete Japanese (47,52). Another use for large iodine doses is in the treatment of thyroid storm where iodine is combined with antithyroid drugs (19).
Iodine and the breast
Breast milk iodine
The role of breast milk as a source of iodine for the neonate is well established (9,53). Breast-milk iodine concentrations vary widely between populations but are highest in colostrum and decrease gradually throughout the lactation period (54). The concentration of iodide in breast milk can be 20- to 30-fold higher than that found in maternal plasma. In addition, the small iodine pool of the neonatal thyroid turns over very rapidly and is highly sensitive to variations in dietary iodine intake (53). Iodine in breast milk mirrors strikethrough varies dietary iodine intake, being lowest mirrors in areas of iodine deficiency with high prevalence of goitre (54,55), increasing when programs of iodine prophylaxis such as salt iodization or administration of iodized oil have been introduced. Although some studies report no difference in iodine intake as measured by UI between breast and bottle fed infants at least in iodine sufficient populations (56), others showed higher values in breast feeding infants suggesting superior iodide transfer (57).
Breast disease
An association between breast cancer incidence and dietary iodine deficiency has long been postulated as breast cancer seemed to be more prevalent in those areas where endemic goitre prevailed (58). Investigators lead by Eskin in the USA (2) suggested that “iodine is a prerequisite for the normal development of breast tissue in higher vertebrates” while Venturi in Italy looking at the extrathyroidal actions of iodide postulated that the antioxidant properties of iodide can contribute to its anti tumour activities in breast, thyroid, and stomach (3). Iodine deficiency has been implicated in breast cancer incidence and mortality as well as in benign breast disease rates while higher iodine intake was believed to be protective (59). Increased thyroid volumes in patients with breast cancer or benign breast disease were also reported (58). The increase in rates of breast cancer with distant metastases in young women in the USA has been attributed in part to a fall in iodine intake (59).
It has been suggested that the low rate of breast cancer reported in Japanese compared to Western women may reflect high dietary iodine intake and specifically seaweed derived iodine as a possible protective factor (48,58). The recent increase in the rate of breast cancer in Japanese women and increasing trends in mortality and incidence patterns of breast cancer have been attributed to the decline in the Japanese diet of seaweed rich foods (48,58). An anticarcinogenic role for iodine or iodine-rich Wakame seaweed was demonstrated in experimental animals who also reported lower tissue iodine levels in rat mammary tumours (60). Lower tissue iodine levels in human breast cancer tissues than in benign breast tumours (fibroadenomas) was demonstrated by the present author’s group (61). Use of radioactive iodine as a potential adjuvant therapy in breast cancers similar to that currently in use in thyroid cancers has been hampered by failure of NIS expression at the cell membrane (62). Efforts to promote NIS trafficking to the breast cell membrane have involved treatment with retinoic acid, oestradiol, IGF or glycosylating promoters (62). Elemental iodine (I2) as distinct from iodide (I−) was suggested as being an active protective factor against breast and other cancers while molecular iodine had beneficial effects on breast pain in fibrocystic breast disease without the antithyroid side effects of iodide therapy (63,64). More recently the group of Aceves using I2 in a pilot study in combination with chemotherapy found that I2 improves the effectiveness of the treatment, decreasing side effects and increasing disease-free survival especially in advanced breast cancer (65).
Iodine and placenta
As with other dietary factors a functioning placenta is necessary for delivering iodine from the mother to the fetus. As no functioning fetal thyroid is present for the first 18–20 weeks of gestation, thyroid hormone related psychoneurological development is entirely dependent on maternal hormone production. When the fetal thyroid develops, its iodine content is extremely low (40–300 µg) compared to approximately 10–20 mg in the adult thyroid but daily thyroid hormone (T4) requirements are much closer: neonate 50 µg; adult 150 µg (6) as neonatal iodine turnover is much more rapid (~17% at birth vs. 1% in adults) (9,53). Studies from the authors laboratory have suggested that the placenta may serve as an iodine storage organ with the iodine content reflecting dietary iodine intake (66). Such storage may compensate for inadequate dietary iodine intake. The concept of placental iodine storage was supported by findings (67) which also identified factors influencing such storage with implications for neonatal health and subsequently on health later in life. Further support for placental iodine storage was provided by the demonstration of a precipitous fall in maternal UI immediately after birth perhaps resulting from placental delivery. Early neonatal UI levels remained roughly equivalent to antenatal maternal values (68).
Iodine and parotid
Another extrathyroidal organ with the ability to concentrate iodide are the parotid and other salivary glands (5). As with the thyroid, salivary gland I- uptake is NIS dependent (18). The exact biologic role of iodide in extrathyroidal tissues, including the salivary gland, is unknown. In this context, it is interesting to note that studies investigating patterns of dental pathology and dietary differences report low rates of dental caries and a lack of tooth loss in populations residing in iodine-rich geographical locations (11). Uptake by the parotid is clinically relevant particularly as parotid damage (sialadenitis) can occur following I131treatment for thyroid cancer or Graves’ disease. Prevention of sialadenitis may require agents to increase saliva flow and thus reduce the time the RAI remains in the salivary glands (69).
Iodine and the skin
Water soluble iodine (povidone iodine, betadine) is frequently used as a skin disinfectant. Apart from its germicidal action on the skin surface, iodine can be both absorbed into and excreted from the skin. Unlike iodide (I−) which is actively taken up by thyroidal NIS, iodine (I2) is believed to be passively absorbed and excreted (13). While a majority of topically applied I2 is lost through evaporation, iodine absorbed through skin has been shown to interfere with I131 uptake in scintigraphy and to diminish the effectiveness of ablative therapy for thyroid cancer (13) and can induce hypothyroidism in infant thyroids. Iodide excreted through the skin during sweating has a published sweat iodine content of 35–40 µg/L and a potential sweat loss of 4–5 L following vigorous exercise, daily iodine losses in sweat equivalent to the WHO-recommended adult daily intake (150 µg) might be expected (70). The combination of sweat loss and urinary excretion of iodine following repeated vigorous exercise if not replaced, would result in a significant diminution in the body’s iodine stores which in the case of elite athletes has the potential of impinging on performance.
Iodine and thyroid cancer
Since both low and high dietary iodine intake can result in increased serum TSH, variations in dietary iodine intake can promote thyroid cellular growth in thyroid cancer. Higher rates of thyroid cancer have been reported in areas of both low and high dietary iodine intake (7,71). There are many factors, such as ethnicity, selenium intake and goitrogen or carcinogen intake that complicate studies linking changes in differentiated thyroid cancer (DTC) rates to iodine intake. An increased prevalence of papillary thyroid cancer has been reported in postpartum Japanese women consuming daily seaweed dishes compared to those whose consumption was 2 days per week or less (72). Iodine deficiency has long been implicated in the pathogenesis of thyroid cancers, particularly follicular thyroid cancer and perhaps the anaplastic variant (71-73). Whatever the origin of diminished or increased iodine intake, anything that diminishes thyroid hormone synthesis or release will inevitably leads to increased TSH and a stimulus to undesirable thyroid cellular growth.
Assessment of iodine intake
There has been a remarkable improvement in dietary iodine intake over recent decades (27). However, the criteria for defining iodine deficiency have been the cause of some debate. Differing methods of assessing iodine excretion include spot urine specimens either single or multiple, in whom either iodine or the iodine creatinine ratio are measured or 24 hour urine collections (9,12). A spot urine specimen in which iodine concentration alone is measured gives satisfactory information on population iodine status, providing a sufficient number of samples are collected (9). Although the WHO has defined deficiency as a median population UI of <100 µg /L, the finding of a single UI measurement <100 µg/L cannot be taken to indicate deficiency. Another criterion is % of individuals in a population having a UI <50 µg/L not exceeding 20% (9,12). As the adult thyroid contains 15–20 mg of iodine, deficiency requires a sustained low iodine intake before iodine stores are depleted (12). Using an arbitrary UI cut off of 100 µg /L has the potential of incorrectly classifying an individual as iodine deficient and perhaps initiating inappropriate iodine prophylaxis with potentially harmful results (12). Another possible pitfall is using data from school aged children (SAC) as milk consumption, a major source of dietary iodine, is typically greater in SAC (10,74). A study of the relationship between estimated iodine requirement (EAR), and recommended dietary allowance (RDA)/recommended nutrient intake (RNI) (12) suggested the use of a UI of 63 µg/L for adult females equivalent to the (RDA) of 95 µg/day or the recommended daily intake of 150 µg/day (95+2× sd 19) corrected to nearest 50 µg (9,12,42). Corresponding values for 10-year-old girls were EAR 72 µg/day equivalent to a UI of 94 µg/L (12).
Iodine in other extrathyroidal tissues
Despite many years of study, the total body iodine remains in dispute. Reports suggest levels of between 15–50 mg (3,12). There is now more agreement on thyroidal iodine which is in the range 15–20 mg (12). Therefore, a considerable amount of body iodine is extrathyroidal. Early work described the presence of iodine in thyroid, breast, placenta, skin, salivary glands, stomach, intestines, kidney, ovary and brain (5). Apart from the thyroid, the function of iodide or iodinated compounds in other tissues remains to be elucidated. An evolutionary role for iodine was suggested (3,75). Iodine as an antioxidant or immunomodulatory agent has been proposed (3,75) and has formed part of the theoretical basis underlying balneotherapy as used in spas (76). An antioxidant role for iodine in biological systems is supported by findings in atmospheric chemistry where gaseous iodine derived from seaweeds acting as an ROS scavenger has been described as the major natural antioxidant in the marine environment (4).
Iodine and plants
The presence of iodine in marine algae (seaweeds) is well established but its equivalent in land-based plants is less well understood. The iodine content of plants and edible vegetables reflect the iodine content of the soil in which they are grown. Plants can take up iodine from soil through the root system but can also be assimilated from the air or absorbed through leaves if dissolved in salt solutions or in rain. This iodine in micromolar quantities can promote growth and yield of crops (77,78). Apart from its natural availability, biofortification with iodine containing irrigation water can enhance the iodine content of plants such as cabbage or tomatoes and significantly contribute to dietary iodine intake (79). Although the exact role of iodine in plants, both aquatic and terrestrial remains unclear, it appears that the antioxidant property identified in seaweeds (4) is shared by land-based plants (79).
Iodine and COVID-19
The use of iodine as an antiseptic exhibiting bactericidal and antiviral properties and in wound healing is well established. These properties led to the exploration of a therapeutic role for iodine in COVID-19 (80), particularly as the high viral load in nasal passages lends itself to topical treatment. A preliminary study involving PVP-1 gargling and nasal spray resulted in negative viral, count by day 3 with virus quantified by PCR before and after treatment (81). An association of COVID 19 infection with atypical subacute thyroiditis (patients with COVID-19) showed evidence of the virus attacking the thyroid (82).
Iodine and the atmosphere
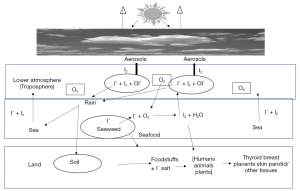
Another role for iodine which does not immediately involve the thyroid is its role in influencing weather patterns in coastal regions through cloud formation. In seaweed abundant coastal area gaseous iodine (I2) released from seaweeds has been shown to contribute to aerosol production and weather patterns through cloud formation affecting climate change (20). Conventional wisdom would suggest that populations living near the sea tended to be iodine replete. However, it has been demonstrated that a higher UI was only observed in those communities living adjacent to an abundant seaweed growth and suggests that the higher concentration of atmospheric gaseous iodine of seaweed origin may have contributed to this increased intake (14). Support for this hypothesis was provided by findings in Portugal and Greece that iodine status was not significantly enhanced by coastal living alone but may reflect local seaweed abundance (23). This finding was not reflected in Tasmania, home to kelp forests, although these have been reported to be in decline (23). Increased UI following immersion in seaweed baths was suggested to involve a mixture of skin absorption and respiration following such treatments (83). Figure 1 shows iodine fluxes between land, sea and lower atmosphere (troposphere). I– from foodstuffs or iodised salt is taken up by the thyroid and other mammalian tissues. In the sea I– is taken up by seaweeds which when under stress reacts with O3 to produce I2 which in the troposphere produces aerosols leading to cloud formation thereby influencing climate change (20,84,85). Marine iodine emissions have been shown to increase in recent times (86).
Conclusions
Continuing clinical and laboratory studies have expanded our knowledge of the involvement of iodine in biological and environmental processes. Although much improvement in dietary iodine supply has been achieved in recent decades, particularly through the increasing availability of iodised salt, iodine deficiency persists and remains a problem particularly for vulnerable groups such as pregnant women, their neonates, and those consuming specialized diets. Iodine supplementation has been very successful, but iodine excess resulting from in ingesting supplements such as kelp tablets or iodine containing substances such as amiodarone, radiological contrast media or topically applied disinfectants require regular monitoring. However, it must be emphasized that huge variations in daily intake can only reflect population rather than individual iodine status. As the prevalence of iodine deficiency in a population depends on the cut off point for UI, methods suggested for a more accurate for a calculated lowering of this cut off are discussed, which in addition to providing a more accurate indication of population iodine deficiency in areas hitherto classified as borderline iodine deficient, will increase the credibility for successful advocacy to other health professionals who profess not to be aware of evidence of any consequences of iodine deficiency in their clinical practices. The possible use of elemental iodine as an adjuvant therapy in breast cancer offers the exciting prospect of expanding the horizons of iodine as a therapeutic agent. The importance of iodine in extrathyroidal tissues such as the breast, placenta, skin, or parotid is discussed as is its role in influencing weather patterns through cloud formation and perhaps iodine intake by respiration in seaweed abundant coastal areas. Although discussion of atmospheric iodine may seem incongruous in a thyroid research journal, the contribution of marine sources to iodine abundance should at least increase consciousness of this major bioavailable source. Hopefully this review, in addition to providing an update on thyroidal and iodine deficiency issues, will increase the appetite for looking at iodine involvement in other tissues and their possible relationship to thyroidal and extrathyroidal disorders.
Acknowledgments
The author acknowledges the support of Professor Niall O’Higgins and the late Mr Enda Mc Dermott of UCD, Dept of Surgery and Professor Colin O’ Dowd of CCAPS, School of Physics, NUI, Galway, Ireland.
Funding: None.
Footnote
Reporting Checklist: The author has completed the Narrative Review reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-21-28/rc
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-21-28/coif). The author is the Editor-in-Chief of the Merck journal, Thyroid International. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kelly FC. Iodine in Medicine and Pharmacy Since its Discovery-1811-1961. Proc R Soc Med 1961;54:831-6. [Crossref] [PubMed]
- Eskin BA. Iodine and mammary cancer. Adv Exp Med Biol 1977;91:293-304. [Crossref] [PubMed]
- Venturi S, Donati FM, Venturi A, et al. Role of iodine in evolution and carcinogenesis of thyroid, breast and stomach. Adv Clin Path 2000;4:11-7. [PubMed]
- Smyth PP. The thyroid and breast cancer: a significant association? Ann Med 1997;29:189-91. [Crossref] [PubMed]
- Brown-Grant K. Extrathyroidal Iodide Concentrating Mechanisms. Physiol Rev 1961;41:189-213. [Crossref]
- Küpper FC, Carpenter LJ, McFiggans GB, et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc Natl Acad Sci U S A 2008;105:6954-8. [Crossref] [PubMed]
- Zimmermann MB. Iodine deficiency. Endocr Rev 2009;30:376-408. [Crossref] [PubMed]
- Hora K. Iodine production and industrial applications. 2016 IDD Newsletter, Aug 2016.
- World Health Organization, United Nations Children’s Fund, and the International Council for the Control of Iodine Deficiency Disorders. Assessment of Iodine Deficiency Disorders and Monitoring their Elimination: A Guide for Programme Managers. 3rd ed. Geneva: World Health Organization 2007 ISBN: 9789241595827.
-
UNICEF Guidance on the Monitoring of Salt Iodization Programmes and Determination of Population Iodine Status 2018 ;11-23. - Jahreis G, Hausmann W, Kiessling G, et al. Bioavailability of iodine from normal diets rich in dairy products--results of balance studies in women. Exp Clin Endocrinol Diabetes 2001;109:163-7. [Crossref] [PubMed]
- Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 2012;70:553-70. [Crossref] [PubMed]
- Nesvadbova M, Crosera M, Maina G, et al. Povidone iodine skin absorption: an ex-vivo study. Toxicol Lett 2015;235:155-60. [Crossref] [PubMed]
- Smyth PP, Burns R, Huang RJ, et al. Does iodine gas released from seaweed contribute to dietary iodine intake? Environ Geochem Health 2011;33:389-97. [Crossref] [PubMed]
- Pesce L, Kopp P. Iodide transport: implications for health and disease. Int J Pediatr Endocrinol 2014;2014:8. [Crossref] [PubMed]
- Tazebay UH, Wapnir IL, Levy O, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med 2000;6:871-8. [Crossref] [PubMed]
- Brown RS. Minireview: developmental regulation of thyrotropin receptor gene expression in the fetal and newborn thyroid. Endocrinology 2004;145:4058-61. [Crossref] [PubMed]
- La Perle KM, Kim DC, Hall NC, et al. Modulation of sodium/iodide symporter expression in the salivary gland. Thyroid 2013;23:1029-36. [Crossref] [PubMed]
- Braverman LE. Iodine and the thyroid: 33 years of study. Thyroid 1994;4:351-6. [Crossref] [PubMed]
- O’Dowd CD, Hoffmann T. Coastal new particle formation: A review of the current state-of-the-art. Environmental Chemistry 2005;2:245-55. [Crossref]
- Peeters RP, Visser TJ. Metabolism of Thyroid Hormone. [Updated 2017 Jan 1]. In: Feingold KR, Anawalt B, Boyce A, et al. editors. Endotext [Internet]. South Dartmouth (MA): 2017 MDText.com, Inc.; 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285545/
- Truesdale V. The biogeochemical effect of seaweeds upon close-to natural concentrations of dissolved iodate and iodide in seawater – Preliminary study with Laminaria digitata and Fucus serratus. Estuarine Coastal and Shelf Science 2008;78:155-65. [Crossref]
- Smyth PPA. Iodine, Seaweed and the Thyroid. Eur Thyroid J 2021;10:101-8. [Crossref] [PubMed]
- National diet and nutrition survey assessment of salt intake from urinary sodium in adults (aged 19 to 64 years) in England, 2018/19. Public Health England.
- Charlton K, Probst Y, Kiene G. Dietary Iodine Intake of the Australian Population after Introduction of a Mandatory Iodine Fortification Programme. Nutrients 2016;8:701. [Crossref] [PubMed]
- Jones E, McLean R, Davies B, et al. Adequate Iodine Status in New Zealand School Children Post-Fortification of Bread with Iodised Salt. Nutrients 2016;8:298. [Crossref] [PubMed]
- Völzke H, Erlund I, Hubalewska-Dydejczyk A, et al. How Do We Improve the Impact of Iodine Deficiency Disorders Prevention in Europe and Beyond? Eur Thyroid J 2018;7:193-200. [Crossref] [PubMed]
- Tayie FA, Jourdan K. Hypertension, dietary salt restriction, and iodine deficiency among adults. Am J Hypertens 2010;23:1095-102. [Crossref] [PubMed]
- Andersson M, Hunziker S, Fingerhut R, et al. Effectiveness of increased salt iodine concentration on iodine status: trend analysis of cross-sectional national studies in Switzerland. Eur J Nutr 2020;59:581-93. [Crossref] [PubMed]
- Eveleigh ER, Coneyworth LJ, Avery A, et al. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020;12:1606. [Crossref] [PubMed]
- Köhrle J, Jakob F, Contempré B, et al. Selenium, the thyroid, and the endocrine system. Endocr Rev 2005;26:944-84. [Crossref] [PubMed]
- Duntas LH. Selenium and the thyroid: a close-knit connection. J Clin Endocrinol Metab 2010;95:5180-8. [Crossref] [PubMed]
- Contempre B, Dumont JE, Ngo B, et al. Effect of selenium supplementation in hypothyroid subjects of an iodine and selenium deficient area: the possible danger of indiscriminate supplementation of iodine-deficient subjects with selenium. J Clin Endocrinol Metab 1991;73:213-5. [Crossref] [PubMed]
- Bath SC, Steer CD, Golding J, et al. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet 2013;382:331-7. [Crossref] [PubMed]
- Chen Y, Guo W, Pan Z, et al. Exploration of the optimal range of urinary iodine concentration in Chinese pregnant women in mildly iodine-deficient and -sufficient areas. Eur J Nutr 2022;61:1221-30. [Crossref] [PubMed]
- Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab 2000;85:3975-87. [PubMed]
- López-Muñoz E, Mateos-Sánchez L, Mejía-Terrazas GE, et al. Hypothyroidism and isolated hypothyroxinemia in pregnancy, from physiology to the clinic. Taiwan J Obstet Gynecol 2019;58:757-63. [Crossref] [PubMed]
- Van Trotsenberg P, Stupa A, Leger A, et al. Congenital Hypothyroidism: A 2020–2021 Consensus Guidelines Update—An ENDO-European Reference Network Initiative Endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid 2021;31:387-419. [Crossref] [PubMed]
- Palmieri N, Forleo M. The potential of edible seaweed within the Western diet. A segmentation of Italian consumers. Int J Gastron Food Sci 2020;100202. [Crossref]
- Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol 2014;10:136-42. [Crossref] [PubMed]
- Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Ann N Y Acad Sci 2019;1446:44-65. [Crossref] [PubMed]
- Institute of Medicine, Academy of Sciences, USA. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Washington, DC: The National Academies Press, 2001.
- European Food Safety Authority EFSA. Summary of Tolerable Upper Intake Levels – version 4 (September 2018) 1 Overview on Tolerable Upper Intake Levels as derived by the Scientific Committee on Food (SCF) and the EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) 2006 ISBN: 92-9199-014-0.
- Petersen M, Knudsen N, Carlé A, et al. Increased Incidence Rate of Hypothyroidism After Iodine Fortification in Denmark: A 20-Year Prospective Population-Based Study. J Clin Endocrinol Metab 2019;104:1833-40. [Crossref] [PubMed]
- Laurberg P. DanThyr 20Years of Iodine Monitoring and Research. Thyroid International 2015;2:1-25.
- Wang C, Li Y, Teng D, et al. Hyperthyroidism Prevalence in China After Universal Salt Iodization. Front Endocrinol (Lausanne) 2021;12:651534. [Crossref] [PubMed]
- Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid 2008;18:667-8. [Crossref] [PubMed]
- Zava TT, Zava DT. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid Res 2011;4:14. [Crossref] [PubMed]
- Dworkin HJ, Jacquez JA, Beierwaltes WH. Relationship of iodine ingestion to iodine excretion in pregnancy. J Clin Endocrinol Metab 1966;26:1329-42. [Crossref] [PubMed]
- Bartalena L, Bogazzi F, Chiovato L, et al. 2018 European Thyroid Association (ETA) Guidelines for the Management of Amiodarone- National Diet and Nutrition Survey Associated Thyroid Dysfunction. Eur Thyroid J 2018;7:55-66. [Crossref] [PubMed]
- l'Allemand D, Grüters A, Beyer P, et al. Iodine in contrast agents and skin disinfectants is the major cause for hypothyroidism in premature infants during intensive care. Horm Res 1987;28:42-9. [Crossref] [PubMed]
- Braverman ER, Blum K, Loeffke B, et al. Managing terrorism or accidental nuclear errors, preparing for iodine-131 emergencies: a comprehensive review. Int J Environ Res Public Health 2014;11:4158-200. [Crossref] [PubMed]
- Delange F, Bourdoux P, Laurence M, et al. Neonatal Thyroid Function in Iodine Deficiency. In: Delange F, Dunn JT, Glinoer D. editors. Iodine Deficiency in Europe. A continuing concern. New York, NY: Plenum Press, 1993:199-209.
- Azizi F, Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin Endocrinol (Oxf) 2009;70:803-9. [Crossref] [PubMed]
- Dror DK, Allen LH. Overview of Nutrients in Human Milk. Adv Nutr 2018;9:278S-94S. [Crossref] [PubMed]
- Nazeri P, Dalili H, Mehrabi Y, et al. Is there any difference between the iodine statuses of breast-fed and formula-fed infants and their mothers in an area with iodine sufficiency? Br J Nutr 2018;119:1012-8. [Crossref] [PubMed]
- Smyth PP, Hetherton AM, Smith DF, et al. Maternal iodine status and thyroid volume during pregnancy: correlation with neonatal iodine intake. J Clin Endocrinol Metab 1997;82:2840-3. [Crossref] [PubMed]
- Smyth PP. The thyroid and breast cancer. Curr Opin Endocrinol Diabetes Obes 2016;23:389-93. [Crossref] [PubMed]
- Rappaport J. Changes in Dietary Iodine Explains Increasing Incidence of Breast Cancer with Distant Involvement in Young Women. J Cancer 2017;8:174-7. [Crossref] [PubMed]
- Funahashi H, Imai T, Tanaka Y, et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)-anthracene-induced mammary tumors in rats. Jpn J Cancer Res 1999;90:922-7. [Crossref] [PubMed]
- Kilbane MT, Ajjan RA, Weetman AP, et al. Tissue iodine content and serum-mediated 125I uptake-blocking activity in breast cancer. J Clin Endocrinol Metab 2000;85:1245-50. [PubMed]
- Kogai T, Brent GA. The sodium iodide symporter (NIS): regulation and approaches to targeting for cancer therapeutics. Pharmacol Ther 2012;135:355-70. [Crossref] [PubMed]
- Eskin BA, Grotkowski CE, Connolly CP, et al. Different tissue responses for iodine and iodide in rat thyroid and mammary glands. Biol Trace Elem Res 1995;49:9-19. [Crossref] [PubMed]
- Aceves C, Anguiano B, Delgado G. Is iodine a gatekeeper of the integrity of the mammary gland? J Mammary Gland Biol Neoplasia 2005;10:189-96. [Crossref] [PubMed]
- Moreno-Vega A, Vega-Riveroll L, Ayala T, et al. Adjuvant Effect of Molecular Iodine in Conventional Chemotherapy for Breast Cancer. Randomized Pilot Study. Nutrients 2019;11:1623. [Crossref] [PubMed]
- Burns R, Azizi F, Hedayati M, et al. Is placental iodine content related to dietary iodine intake? Clin Endocrinol (Oxf) 2011;75:261-4. [Crossref] [PubMed]
- Neven KY, Marien CBD, Janssen BG, et al. Variability of iodine concentrations in the human placenta. Sci Rep 2020;10:161. [Crossref] [PubMed]
- Smyth PP, Smith DF, Sheehan S, et al. Short-term changes in maternal and neonatal urinary iodine excretion. Thyroid 2007;17:219-22. [Crossref] [PubMed]
- Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid 2003;13:265-71. [Crossref] [PubMed]
- Smyth PP, Duntas LH. Iodine uptake and loss-can frequent strenuous exercise induce iodine deficiency? Horm Metab Res 2005;37:555-8. [Crossref] [PubMed]
- Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8. [Crossref] [PubMed]
- Michikawa T, Inoue M, Shimazu T, et al. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev 2012;21:254-60. [Crossref] [PubMed]
- Smyth PP. Iodine and anaplastic thyroid carcinoma. Thyroid 2010;20:581-2. [Crossref] [PubMed]
- Lazarus JH. Monitoring iodine nutritional status: adults or schoolchildren? J Endocrinol Invest 2021;44:383-5. [Crossref] [PubMed]
- Aceves C, Mendieta I, Anguiano B, et al. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. Int J Mol Sci 2021;22:1228. [Crossref] [PubMed]
- Winkler R. Iodine—A Potential Antioxidant and the Role of Iodine/Iodide in Health and Disease. Natural Science 2015;07:548-57. [Crossref]
- Kiferle C, Martinelli M, Salzano AM, et al. Evidences for a Nutritional Role of Iodine in Plants. Front Plant Sci 2021;12:616868. [Crossref] [PubMed]
- Dobosy P, Vetési V, Sandil E. Effect of Irrigation Water Containing Iodine on Plant Physiological Processes and Elemental Concentrations of Cabbage (Brassica oleracea L. var. capitata L.) and Tomato (Solanum lycopersicum L.) Cultivated in Different Soils. Agronomy 2020;10:720. [Crossref]
- Hong CL, Weng HX, Qin YC, et al. Transfer of iodine from soil to vegetables by applying exogenous iodine. Agron Sustain Dev 2008;28:575-83. [Crossref]
- Verheesen RH, Traksel RAM. Iodine, a preventive and curative agent in the COVID-19 pandemic? Med Hypotheses 2020;144:109860. [Crossref] [PubMed]
- Guenezan J, Garcia M, Strasters D, et al. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg 2021;147:400-1. [Crossref] [PubMed]
- Muller I, Cannavaro D, Dazzi D, et al. SARS-CoV-2-related atypical thyroiditis. Lancet Diabetes Endocrinol 2020;8:739-41. [Crossref] [PubMed]
- Westby T, Cadogan A, Duignan G. In vivo uptake of iodine from a Fucus serratus Linnaeus seaweed bath: does volatile iodine contribute? Environ Geochem Health 2018;40:683-91. [Crossref] [PubMed]
- Legrand M, McConnell JR, Preunkert S, et al. Alpine ice evidence of a three-fold increase in atmospheric iodine deposition since 1950 in Europe due to increasing oceanic emission. PNAS 2018;115:12136-41. [Crossref] [PubMed]
- Cuevas CA, Maffezzoli N, Corella JP, et al. Rapid increase in atmospheric iodine levels in the North Atlantic since the mid-20th century. Nat Commun 2018;9:1452. [Crossref] [PubMed]
- Carpenter LJ, Chance RJ, Sherwen T, et al. Marine iodine emissions in a changing world. Proc Math Phys Eng Sci 2021;477:20200824. [Crossref] [PubMed]
Cite this article as: Smyth PPA. Narrative review: iodine—thyroidal and extrathyroidal actions. Ann Thyroid 2022;7:4.


