
Hungry bone syndrome after thyrotoxicosis factitia complicated with thyroid storm: a case report
Introduction
The hungry bone syndrome (HBS) is reported as a well-established thyroidectomy complication of in Graves’ disease, especially in the case of severe thyrotoxicosis. This phenomenon is caused by a rapid increase in the skeletal uptake of blood calcium leading to persistent symptomatic hypocalcemia (1,2). The severity of hypocalcemia would depend on the severity of the thyrotoxicosis, previous low bone mass density (BMD), and the degree of the elevated preoperative serum alkaline phosphatase (ALP) (3). Although the incidence is still unclear, there have been many observational trials and case reports (3-5). Apart from post-thyroidectomy hypoparathyroidism and reversal of the high bone turnover in Graves’ disease, other etiologies of hypocalcemia after thyrotoxicosis treatment have been rarely reported. To our knowledge, there have been no case reports of hypocalcemia originating from the HBS after thyrotoxicosis factitia treatment.
Therefore, we aimed to report the first case of thyrotoxicosis factitia presenting a thyroid storm complicated by profound hypocalcemia after discontinuing the offending drug. Hence, we present this case in accordance with the CARE reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-21-18/rc).
Case presentation
Clinical history
A 56-year-old married woman with a history of intermittent treatment of bipolar disorder and body dysmorphism was admitted to our hospital with thyrotoxicosis factitia in 2017. She had been previously healthy. Her condition was diagnosed by a history of taking diet pills (identified as levothyroxine tablets) and signs of thyrotoxicosis. A laboratory investigation showed incomplete suppression of thyroid-stimulating hormone (TSH), very high value of free thyroxine (FT4) compared to free triiodothyronine (FT3), and a markedly decreased level of serum thyroglobulin (Tg). She was treated with cholestyramine and dexamethasone and was discharged in a full recovery condition. Then, the patient became lost in the follow-up shortly after being discharged.
Two years later, in 2019, the patient presented herself at the Emergency Department with alteration of consciousness and shortness of breath with signs of thyrotoxicosis, which was similar to the first episode. We were informed that the patient had again taken large indeterminate doses of levothyroxine tablets as diet pills for a four-month duration and did not do the follow-up with her psychiatrist.
Physical examination
On admission, the patient was 155 centimeters (cm) tall and weighed 43 kilograms (kg), with a body mass index (BMI) of 17.92 kg/m2. She lost 6 kg in four months (changes in bodyweight of 49 to 43 kg and BMI of 20.42 to 17.92 kg/m2). The physical examination at the Emergency Department revealed an irregular heart rate of 169 beats per minute, rapid and shallow breathing patterns at a rate of 28–32 per minute, and fine crepitation in both lower lung areas. The thyroid gland was only around 10 grams, almost non-palpable, and without thyroid bruit. She had tremors of both hands and sweating palms.
Laboratory and radiographic investigations
The laboratory data were as follows: TSH <0.005 µIU/mL (0.27–4.20 µIU/mL), FT4 >7.77 ng/dL (0.93–1.70 ng/dL), FT3 24.57 pg/mL (2.0–4.40 pg/mL), serum Tg 1.42 ng/mL (3.50–77 ng/mL), antithyroglobulin antibody 0.77 IU/mL (<4.11 IU/mL), and TSH receptor antibody <0.8 IU/L (<1.75 IU/L). The albumin corrected calcium, ALP, and serum creatinine values were in a normal range (Tables 1,2). The chest radiography showed cardiomegaly with pulmonary congestion. Acute respiratory failure was diagnosed, and the patient was immediately intubated, assisted with mechanical ventilation, and transferred from the Emergency Department to the intensive care unit (ICU). The Burch and Wartofsky score (BWS) was 80, which was highly suggestive of a thyroid storm. The bedside thyroid color-flow Doppler sonography revealed a normal size thyroid gland with decreased blood flow. The echocardiography showed cardiomyopathy with reduced left ventricular ejection fraction (LVEF) to 42%, despite normal coronary angiography (CAG) afterward. As a result of the clinical history and investigation, again thyrotoxicosis factitia was diagnosed, but this episode was additionally complicated with a thyroid storm.
Table 1
| Laboratory parameters | Results |
|---|---|
| Hematology | |
| White blood cell (cell/mm3) | 11,360 |
| Hemoglobin (g/dL) | 10.2 |
| Platelet (×103/mm3) | 217 |
| Blood chemistry | |
| Glucose (mg/dL) | 79 |
| Creatinine (0.5–1 mg/dL) | 0.67 |
| Sodium (135–145 mEq/dL) | 137.9 |
| Potassium (3.5–4.5 mEq/dL) | 3.82 |
| Carbon dioxide (21–31 mEq/dL) | 19 |
| Calcium/albumin corrected calcium (8.8–10.6 mg/dL) | 9.8/10 |
| Phosphate (2.5–4.5 mg/dL) | 2.8 |
| Albumin (3.5–4.5 g/dL) | 3.74 |
| Magnesium (1.8–2.6 mg/dL) | 2.0 |
| ALP (30–120 IU/L) | 119 |
| 25(OH) vitamin D (≥30 ng/dL) | 8.5 |
| Thyroid autoantibody | |
| Anti-thyroglobulin (<4.11 IU/mL) | 0.77 |
| TSH receptor antibody (<1.75 IU/L) | <0.8 |
| Tg (3.50–77 ng/mL) | 1.42 |
Units and normal ranges are provided in parentheses. ALP, alkaline phosphatase; 25(OH) vitamin D, 25-hydroxy vitamin D; TSH, thyroid-stimulating hormone; Tg, thyroglobulin.
Table 2
| Laboratory value | Day 0 | Day 1 | Day 3 |
|---|---|---|---|
| TSH (0.27–4.2 µIU/mL) | <0.005 | 0.006 | 0.024 |
| FT3 (2–4.4 pg/mL) | 24.57 | 2.3 | 1.64 |
| FT4 (0.93–1.7 ng/dL) | >7.77 | 7.16 | 2.51 |
Units and normal ranges are provided in parentheses. TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; FT4, free thyroxine.
Management of thyroid crisis
The offending drug (levothyroxine) was immediately stopped, and intravenous dexamethasone 4 mg was initiated every 12 hours with the aim to decrease the peripheral conversion of thyroxine. Propranolol was not prescribed according to her active low-output heart failure on top of the thyrotoxic dilated cardiomyopathy. Seventy-two hours after admission to the ICU, the patient developed flank pulmonary edema with cardiogenic shock. The cardiologist initiated dobutamine as an inotropic agent to rescue the hemodynamics. The cardiorenal syndrome subsequently occurred; thus, emergent continuous renal replacement therapy was also initiated on the seventh day of admission. After intensive organ support therapy and treatment of the thyroid crisis, the patient’s FT3 and FT4 levels dramatically declined to a near-normal range (Table 2), and the patient gained self-consciousness, so we discontinued dexamethasone on the fourth day of admission. The patient also resumed her normal renal function and discontinued renal replacement therapy within one week.
Clinical progression and HBS
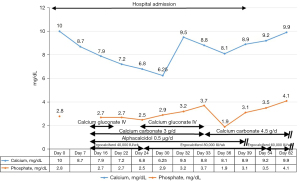
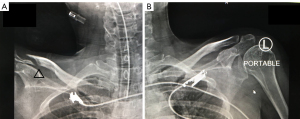
Two weeks after admission, the patient complained of a tingling sensation and muscle cramps. Her serum calcium values gradually decreased to as low as 6.25 mg/dL (8.8–10.6 mg/dL), the serum phosphate level was 2.5 mg/dL (2.5–4.5 mg/dL), serum magnesium level was 1.3 mg/dL (1.8–2.6 mg/dL), and ALP level increased to 139 IU/L (30–120 IU/L) (Figure 1). The intact parathyroid hormone level was increased to 78 pg/mL (15–68 pg/mL) (measured when the calcium value was 8.8 mg/dL). The plain radiograph, thoroughly reviewed by the radiologist, revealed a markedly advanced-than-age radiolucency of both distal clavicles and non-union right distal clavicular fracture without appropriate callus, which was referred to as thyrotoxic osteodystrophy (Figure 2). The HBS was diagnosed and calcium gluconate was administered intravenously since early calcium descent. After 10 days of intravenous infusion of calcium gluconate (2,500 mg of calcium gluconate per day on average, equivalent to 225 mg of elemental calcium) together with oral administration of precipitated calcium carbonate 3 g/day (equivalent to 1,200 mg of elemental calcium per day), ergocalciferol 40,000 IU/week, and alphacalcidol 0.5 µg/day, the serum calcium level, paresthesia, and cramping eventually ameliorated (Figure 1). Throughout the hospital admission, the patient strictly needed oral calcium and vitamin D supplement. Tapering both agents had been tried but failed due to recurrent symptomatic hypocalcemia. Finally, two months after being discharged from hospital, the patient gained her normal weight (49–50 kg) with clinical euthyroidism. The serum calcium, phosphate, and ALP returned to normal levels, so oral administration of calcium, ergocalciferol, and alphacalcidol was successfully reduced and eventually discontinued without any adverse events (Figure 1). All of the calcium values in this report were displayed as corrected calcium by Payne’s formula; in the case of low albuminemia (<4.0 g/dL), the corrected calcium (mg/dL) was calculated by total calcium (mg/dL) + 0.8 × [4.0 − albumin (g/dL)] (6).
After successful calcium and vitamin D discontinuation, the patient again shortly lost contact with our clinic and her psychiatrist; therefore, we could not obtain her informed consent. We nevertheless reassured for non-personally identifiable patient’s specific information throughout the report. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013).
Discussion
To the best of our knowledge, this was the first case of hypocalcemia resulting from HBS after thyrotoxicosis factitia treatment. The patient had been exposed to a large dose and long duration of levothyroxine enough to produce thyrotoxic changes in many organ systems, including bone and mineral metabolism. There were three obvious clinical clues. Firstly, the patient already had thyrotoxic osteodystrophy, which was the most important risk factor of developing HBS (3,7,8). Secondly, the patient had thyrotoxicosis factitia with thyroid crisis, rarely reported, but associated with the massive amount and, especially, longer duration of levothyroxine abuse (1,9,10). Lastly, the patient already had thyrotoxic dilated cardiomyopathy with acute low-output heart failure, which was strongly associated with untreated long-standing and severe thyrotoxicosis (11).
Thyrotoxic dilated cardiomyopathy is a rare condition accounting for less than 1% of initial thyrotoxicosis presentation (12) of which the hyperadrenergic state compensates in maintaining the cardiac output. This raised the concern of using beta-blockers in the management of the active low-output heart failure in thyrotoxic dilated cardiomyopathy, which could possibly halt this compensation and lead to abruptly reduced cardiac output and, consequently, hemodynamic instability (13).
Thyrotoxicosis is one of the established causes of increased bone turnover, both bone resorption and formation (14). In the state of thyrotoxicosis, excess T3 binds to the thyroid hormone receptor alpha-1 subtype in the nucleus of both the osteoblasts and osteoclasts and activates new shortening remodeling cycles (14). The remodeling cycle even shows an imbalance between the resorption and formation that leads to a net loss of approximately 10% of bone per cycle (15). This would eventually result in a decrease in bone mass and increase fragility fracture in thyrotoxicosis patients on both the trabecular and cortical bones, referred to as thyrotoxic osteodystrophy (3,14,16). The prospective study revealed subsequent significant improvement of BMD during 18±3 months of antithyroid treatment (17) and the fracture risk returned to normal more than one year afterward (16).
The calcium derangement in thyrotoxicosis, owing to the bone remodeling effect, was also a concerning issue. Treating with antithyroid medication, the thyroid hormone levels gradually decreased to a euthyroid state; thus, the high normal total serum level of calcium and urinary calcium excretion at diagnosis decreased without any symptoms, and the serum PTH level slightly increased during the follow-up (17). In contrast, the sudden decline of thyroid hormones would lead to rapid reversal of bone turnover and, consequently, a rapid skeletal uptake of calcium from the serum, thus causing hypocalcemia (3,7). This phenomenon was occasionally complicated with prolonged hypocalcemia with a simultaneous fall in the serum phosphorus and magnesium levels but with an appropriate increase in the PTH level, specifically called HBS (3,7,8). The clinical course of HBS would depend on the etiology of the excess bone remodeling state. In primary hyperparathyroidism, transient hypocalcemia occurs within 18 hours after parathyroidectomy and remains low up to several months (18). In thyrotoxic osteodystrophy, transient hypocalcemia is less severe and starts at 72- or 96-hour post-thyroidectomy and may last for up to 12 weeks (7,19).
Interestingly, this patient showed gradually progressive hypocalcemia, hypophosphatemia, and hypomagnesemia, defined as HBS, which started in the second week and peaked at the fourth week after the discontinuation of the offending drug. There were also case reports of HBS post thyrotoxicosis treatment (antithyroid medication, radioactive iodine therapy, and subtotal thyroidectomy) of which the onset beyond 96 hours, varied between 2 to 6 months (5,8,19) (Table 3). All of them concordantly had thyrotoxic osteodystrophy at the diagnosis, which was defined by osteopenia in either a plain radiograph or BMD. The presumably rather slow decline of the thyroid hormones after the treatment, compared to typical HBS post total thyroidectomy, may contribute to this phenomenon.
Table 3
| Age (y)/ethnicity/sex | Thyrotoxicosis etiology | Duration of thyrotoxicosis (mo) | Definite thyrotoxicosis treatment | Onset of HBS since definite treatment (mo) | Serum Ca (mg/dL) | Serum PO4 (mg/dL) | Serum Mg (mg/dL) | Serum PTH (pg/mL) | Serum ALP (IU/L) | Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Medication | Duration of Ca supplement (mo) | ||||||||||
| 54/Chinese/F (5) | Recurrent Graves’ disease (partial thyroidectomy 4 years) | 7 | Radioactive iodine therapy | 5 | 4.01 (8.42–10.42) | 8.24 (2.48–4.49) | 1.56 (1.53–2.28) | 48 (35–90) | 717 (30–120) | · Ca carbonate · Mg sulfate · Vitamin D2 |
22 (until the last follow-up) |
| 61/American/F (19) | NA | 2 | Radioactive iodine therapy | 6 | 8.3 (NA) | NA | NA | 105.2 (10–65) | 239.95 (NA) | · Ca carbonate | 3 |
| 25/Chinese/F (8) | Graves’ disease | NA | Subtotal thyroidectomy | 2 | 7.5 (8.34–11.22) | 5.42 (2.79–4.15) | NA | 89.92 (15–65) | 540 (0–150) | · Ca carbonate · Calcitriol |
3 |
| 55/Thai/F† | Thyrotoxicosis factitia | 4 | Discontinue offending drug | 1 | 6.25 (8.8–10.6) | 2.5 (2.5–4.5) | 1.3 (1.8–2.6) | 78 (15–68) | 139 (30–120) | · Ca carbonate · Vitamin D2 · Alphacalcidol |
2 |
†, our case. HBS, hungry bone syndrome; y, years old; mo, months; Ca, calcium; PO4, phosphorous; Mg, magnesium; PTH, parathyroid hormone; ALP, alkaline phosphatase; F, female; NA, not available.
Additionally, a gradual decline of the tissue thyroid hormone might play a role in the prolonged onset of HBS in this patient. The pharmacokinetic study in a patient with massive levothyroxine ingestion illustrated that the serum T4 half-life declined from seven days in euthyroid individuals to 5.7 days; conversely, the half-life of serum T3 was apparently prolonged from 25 hours to 5.3 days, which was explained by continuous conversion from a large dose of levothyroxine (20). The unmeasurable tissue T3, which was the better clinical predictor of thyrotoxicosis factitia, was supposed to have an even longer half-life than serum T3 (21,22). The timing of 3 to 4 weeks was also equivalent to five half-lives of the massive elimination of levothyroxine.
Other factors which may confound persistent hypocalcemia were the previous depletion of the vitamin D level, concomitant critical illness, and acute kidney injury. The vitamin D deficiency might play a role in mild hyperparathyroidism and hypocalcemia in this patient, not only the HBS itself. The critical illness and acute kidney injury would lead to malabsorption of intestinal calcium, impaired parathyroid hormone secretion and action, and impaired vitamin D synthesis (23,24). Nevertheless, these conditions could not entirely explain hypocalcemia with HBS in this patient.
Our obvious limitations were the lack of the comprehensive pre- and post-treatment BMD study and serum bone markers, i.e., osteocalcin and bone-specific ALP. This was, because to our knowledge, there was still a lack of normal cut-off levels for patients with acute kidney injury (25).
Conclusions
In conclusion, we reported the first case of thyrotoxicosis factitia with thyroid storm complicated by HBS after treatment. Therefore, it was recommended that close monitoring of calcium and bone mineral metabolism in patients under treatment for thyrotoxicosis factitia should be undertaken. When HBS occurs, prompt adequate supplementation of intravenous calcium, oral calcium, and vitamin D should be conducted until eucalcemia is achieved.
Acknowledgments
We thank Mr. Andrew Barclay for his effort in linguistic revision of this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-21-18/rc
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-21-18/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-21-18/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. After successful calcium and vitamin D discontinuation, the patient again shortly lost contact with our clinic and her psychiatrist; therefore, we could not obtain her informed consent. We nevertheless reassured for non-personally identifiable patient’s specific information throughout the report. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid 2016;26:1343-421. [Crossref] [PubMed]
- Oltmann SC, Brekke AV, Schneider DF, et al. Preventing postoperative hypocalcemia in patients with Graves disease: a prospective study. Ann Surg Oncol 2015;22:952-8. [Crossref] [PubMed]
- Kusuki K, Mizuno Y. Hungry bone syndrome after thyroidectomy for thyroid storm. BMJ Case Rep 2019;12:231411. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta-analysis of predictors of post-thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Dembinski TC, Yatscoff RW, Blandford DE. Thyrotoxicosis and hungry bone syndrome--a cause of posttreatment hypocalcemia. Clin Biochem 1994;27:69-74. [Crossref] [PubMed]
- Payne RB, Little AJ, Williams RB, et al. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J 1973;4:643-6. [Crossref] [PubMed]
- Karunakaran P, Maharajan C, Ramalingam S, et al. Is hungry bone syndrome a cause of postoperative hypocalcemia after total thyroidectomy in thyrotoxicosis? A prospective study with bone mineral density correlation. Surgery 2018;163:367-72. [Crossref] [PubMed]
- Guo Z, Zhao L, Xie Y, et al. Hungry Bone Syndrome Secondary to Subtotal Thyroidectomy in A Patient With Thyrotoxicosis. Am J Med Sci 2021;362:314-20. [Crossref] [PubMed]
- Yoon SJ, Kim DM, Kim JU, et al. A case of thyroid storm due to thyrotoxicosis factitia. Yonsei Med J 2003;44:351-4. [Crossref] [PubMed]
- Allen KM, Crawford VB, Conaglen JV, et al. Case report: clues to the diagnosis of an unsuspected massive levothyroxine overdose. CJEM 2015;17:692-8. [Crossref] [PubMed]
- Klein I, Danzi S. Thyroid disease and the heart. Circulation 2007;116:1725-35. [Crossref] [PubMed]
- Dahl P, Danzi S, Klein I. Thyrotoxic cardiac disease. Curr Heart Fail Rep 2008;5:170-6. [Crossref] [PubMed]
- Abubakar H, Singh V, Arora A, et al. Propranolol-Induced Circulatory Collapse in a Patient With Thyroid Crisis and Underlying Thyrocardiac Disease: A Word of Caution. J Investig Med High Impact Case Rep 2017;5:2324709617747903. [Crossref] [PubMed]
- Bassett JH, Williams GR. Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr Rev 2016;37:135-87. [Crossref] [PubMed]
- Williams GR, Bassett JHD. Thyroid diseases and bone health. J Endocrinol Invest 2018;41:99-109. [Crossref] [PubMed]
- Vestergaard P, Mosekilde L. Hyperthyroidism, bone mineral, and fracture risk--a meta-analysis. Thyroid 2003;13:585-93. [Crossref] [PubMed]
- Belsing TZ, Tofteng C, Langdahl BL, et al. Can bone loss be reversed by antithyroid drug therapy in premenopausal women with Graves' disease? Nutr Metab (Lond) 2010;7:72. [Crossref] [PubMed]
- Witteveen JE, van Thiel S, Romijn JA, et al. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol 2013;168:R45-53. [Crossref] [PubMed]
- Grieff M. The hungry bone syndrome after medical treatment of thyrotoxicosis. Ann Intern Med 2003;139:706-7. [Crossref] [PubMed]
- Shilo L, Kovatz S, Hadari R, et al. Massive thyroid hormone overdose: kinetics, clinical manifestations and management. Isr Med Assoc J 2002;4:298-9. [PubMed]
- Majlesi N, Greller HA, McGuigan MA, et al. Thyroid storm after pediatric levothyroxine ingestion. Pediatrics 2010;126:e470-3. [Crossref] [PubMed]
- Nygaard B, Saedder EA, Dalhoff K, et al. Levothyroxine Poisoning - Symptoms and Clinical Outcome. Basic Clin Pharmacol Toxicol 2015;117:280-5. [PubMed]
- Kelly A, Levine MA. Hypocalcemia in the critically ill patient. J Intensive Care Med 2013;28:166-77. [Crossref] [PubMed]
- Leaf DE, Christov M. Dysregulated Mineral Metabolism in AKI. Semin Nephrol 2019;39:41-56. [Crossref] [PubMed]
- Nizet A, Cavalier E, Stenvinkel P, et al. Bone alkaline phosphatase: An important biomarker in chronic kidney disease - mineral and bone disorder. Clin Chim Acta 2020;501:198-206. [Crossref] [PubMed]
Cite this article as: Kaewkrasaesin C, Srichomkwun P. Hungry bone syndrome after thyrotoxicosis factitia complicated with thyroid storm: a case report. Ann Thyroid 2022;7:2.


