
Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: first Australasian case report
Introduction
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) is a rare tumour of the thyroid gland. It was first described by Chan et al. (1) in 1991. The histological features of SMECE include nests or strands of epidermoid or squamoid tumour cells, rare mucous cells, a prominent sclerotic stroma with eosinophilic and lymphocytic infiltration in a setting of chronic lymphocytic (Hashimoto’s) thyroiditis (1-6). SMECE occurs predominantly in females and the age ranges from 32 to 89 with an average age in the mid-50’s (2,3). No standard of care is currently defined for SMECE of the thyroid gland but usually includes surgery and/or adjunctive therapy. All are considered when outlining the treatment plan in the multidisciplinary team setting. We report this rare pathology in a patient who was successfully treated in accordance with the CARE reporting checklist (available at https://aot.amegroups.com/article/view/10.21037/aot-21-17/rc).
Case presentation
A 59-year-old female lifelong non-smoker was referred to the ear, nose and throat (ENT) clinic with a seven-week history of a growing neck lump with dyspnoea, orthopnoea and recurrent right otalgia with yawning. Medical history includes a right branchial cyst excision in 2003 with no personal history of radiation exposure, hypothyroidism or hyperthyroidism and no family history of thyroid disease. Examination revealed a visibly enlarged thyroid gland with no overlying skin changes. There was a palpable mass over the left thyroid lobe and isthmus extending to left neck level IV. It was mobile on palpation and on swallowing. No abnormality of the right thyroid gland was palpable. To palpate the neck, levels I, II, III, V bilaterally and level IV on the right were normal. Flexible nasendoscopy showed normal functioning vocal cords.
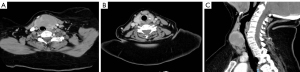
Computed tomography (CT) scan revealed a large nodule in the left thyroid lobe extending into the isthmus measuring 57×40×39 mm. There are ill-defined anterior margins of the nodule with possible extrathyroidal spread. Associated cluster of level VI enlarged lymph nodes and multiple enlarged nodes within level IV/Vc (Figure 1).
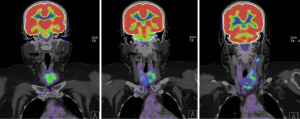
PET-CT showed 18F-fluorodeoxyglucose (FDG) avidity of the thyroid mass centred in the left thyroid lobe SUV 2.9. Left level II, III and IV lymphadenopathy with nodes measuring up to 19×12 mm and left level VI lymphadenopathy with nodes measuring up to 20×16 mm. No right sided cervical lymphadenopathy. No distant disease evident on PET-CT (Figure 2).
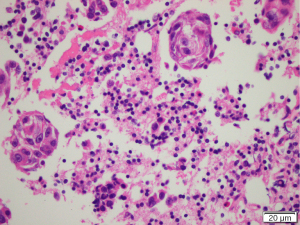
Fine needle aspiration was undertaken (Figure 3). There was a background of inflammatory cells mainly lymphoid in nature with small numbers of eosinophils, plasma cells and histiocytes. Groups of cells with prominent irregular nuclei and macronucleoli with irregular chromatin with some clearing and abundant cytoplasm. The immunophenotype was consistent with a squamous cell carcinoma (SCC). Thyroglobulin level was <0.10 µg/L. Flow cytometry was suggestive of a reactive lymphoid population.
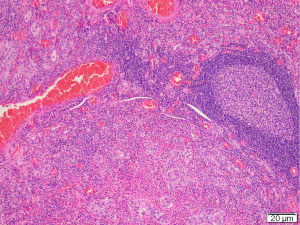
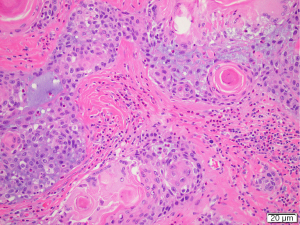
The case was discussed at the regional head and neck cancer meeting (HNC MDM) and consensus recommendation was to proceed with surgery. The patient underwent total thyroidectomy, selective left neck dissection of levels IIa/b, III, IV and V and central dissection of levels VI and VII. Surgical findings included clinical tethering of the thyroid gland to left sided sternothyroid muscles and the left sided strap muscles were resected as part of the thyroid specimen. The recurrent laryngeal nerve was identified and preserved. Berry’s ligament was dissected with no evidence of disease extension laterally. Postoperative hypoparathyroidism with hypocalcaemia was managed with oral calcium and calcitriol supplementation. PTH recovered within six weeks. Postoperative histology was primarily reported as Hashimoto’s thyroiditis with atypical squamous metaplasia. Review of the histology was undertaken at the HNC MDM. Concluding results were of an SMECE of the left and right thyroid lobes 53×45×42 mm with no extracapsular extension. There was no lymphovascular or perineural invasion and the background was in keeping with prominent Hashimoto’s thyroiditis (Figures 4,5). The surrounding nodes were reactive. This resulted in a final diagnosis of pT3aN0 SMECE confined to the thyroid with 0/35 nodes. BRAF and MAML2 mutation analysis was negative. Genetic testing for EGFR, KIT, KRAS and NRAS mutations was negative. Further discussion at the regional HNC MDM concluded on-going observations with close clinical follow up and limited benefit for radioactive iodine or radiotherapy given complete excision of the tumour without nodal involvement.
Patient perspective
I visited my local GP about an ear infection which was very painful, and also asked about the lump that I had become aware of at the front of my neck. I had a great GP who immediately referred me to a specialist and was proactive about following up. It all took quite a while, 4 months from seeing the GP to having the scan, but after the scan results everything happened very quickly. The following week I had an appointment with the surgeon for a biopsy, the week after that a PET scan, the week after that the multi-disciplinary meeting, and then the next week the surgery. The surgeon and all the team were fantastic—so professional, so kind—I felt in good hands and very grateful to be treated with such expertise and attention at every stage. I had a few weeks in hospital and, again, all the team were amazing—doctors, nurses, phlebotomists… I am full of the utmost respect.
I heard that the identification of the type of cancer was done by quite a few different pathologists and the fact that the exact and rare diagnosis was pursued so assiduously is a real credit to them all. I recovered really well from the surgery, and the neat scar from one side of my neck to the other is, I’ve been told, nothing that a nice diamond necklace wouldn’t hide! The most anxious time—dark thoughts in the middle of the night—was waiting to find out what sort of cancer it was, but I am so fortunate to have such a supportive husband and close family that wrapped me in positivity and caring. It was an enormous relief that the tumour had been entirely removed, and that no further cancer treatment was necessary. I am glad to be monitored so carefully, on-going, and feel so lucky to have come through this chapter of my life so well.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
To our knowledge, this case reports the first case of SMECE in Australasia. SMECE is a rare tumour of the thyroid gland that predominantly occurs in females in the age range of 32 to 89 (2). This case history highlights the difficulty of reaching this diagnosis and the need to consider SMECE as a differential diagnosis for the finding of squamous cell abnormalities on fine needle aspiration of the thyroid.
This case presents clinically like previously described cases of SMECE in the literature. In the original article by Chan et al. (1) in 1991, all eight cases presented with a thyroid nodule and euthyroidism with no peripheral blood eosinophilia. A 2016 literature review by Shah et al. (5) found that 36/45 (80%) of cases presented with a painless mass and 4/45 (9%) had compressive symptoms; 44/50 (88%) of cases were female with a median age of 56.1 years (5). The most common tumour location was in the lateral lobe 38/43 (88%) of cases while 4/43 (9%) showed diffuse involvement (5). This 59-year-old female presented in a similar manner with a painless thyroid mass and demonstrated compressive symptoms.
The management of our case was limited by the difficulty in obtaining a diagnosis of SMECE from fine needle aspiration alone. This resulted in an initial diagnosis of SCC of the thyroid gland. Retrospectively, review of the FNA reported presence of eosinophils, which would not be expected to be found in thyroid SCC and may have directed differential diagnosis towards SMECE (1,7). The strengths of this case report show the importance of considering SMECE as a differential diagnosis if SCC is considered. This case report highlights the significant range of differential diagnoses possible. The strengths of this case also show the importance of discussion and review at multidisciplinary meetings for the management of cancer patients.
The histological characteristics of SMECE are epidermoid tumour cells with squamous differentiation that form nests or strands. These are interspersed with a sclerotic stroma which is infiltrated with rare mucous cells, eosinophilic and lymphocytic infiltration and is almost always associated with chronic lymphocytic thyroiditis in the non-neoplastic portion of the thyroid gland (1-5,7). Immunohistochemical stain testing is predominantly negative for thyroglobulin and positive for CK, p63 and mucin (5,6). TTF-1 expression is variable (5). Some genetic mutations have been associated with SMECE including BRAF although this was negative in our case (6).
The fine needle aspiration immunophenotype of our case was suggestive of SCC. Microscopic findings of SCC show increased nuclear atypia and pleomorphism with numerous mitoses and frequent necrosis whilst lacking the finding of lymphocytes and eosinophils that are seen in SMECE (1,7). SCC clinically presents very similar to anaplastic carcinoma with aggressive growth and a poor prognosis. Anaplastic carcinoma has a diffuse growth, increased nuclear atypia, associated necrosis and has a neutrophilic infiltrate rather than lymphocytes and eosinophils (1,7). Papillary thyroid carcinoma (PTC) has characteristic findings of nuclear clearing, nuclear grooves and intranuclear cytoplasmic inclusions (7). Other characteristic features include follicles, papillae, psammoma bodies and thyroglobulin immunoreactivity of which SMECE lacks (1). PTC does not feature tissue eosinophilia (1); 90% of mutations for PTC have been discovered (5). None of these mutations have been detected in SMECE (5). Nodular sclerosing Hodgkin’s lymphoma (NS-HL) shares some features with SMECE including an inflammatory milleu of lymphocytes and eosinophils, sclerosis with broad collagen bands and fibrotic tissue (8,9). However, SMECE lacks the diagnostic Reed-Sternberg cells of NS-HL (9). Salivary mucoepidermoid carcinoma (MEC) to the thyroid is also a rare entity. The histological findings resemble mucin-containing cysts with solid epithelial nests (10). The background is usually of normal thyroid tissue without Hashimoto’s thyroiditis (10). Histological appearances differ despite the similar nomenclature of the tumours. MEC mutational analysis can show MAML2 mutation (10). Again MEC does not appear to be associated with eosinic infiltration (10). SMECE occurs in the setting of Hashimoto’s thyroiditis in the non-neoplastic thyroid gland (2). SMECE should be considered in the differential diagnosis for findings of Hashimoto thyroiditis with regions of fibrosis and squamous metaplasia (3). Definitive cytologic diagnosis on pre-operative FNA or biopsy of SMECE is difficult due to variability in cellularity and the non-specific nature of findings (3). It should be kept in the differential diagnosis particularly if clinical findings are not in keeping with Hashimoto thyroiditis alone.
There are no evidence-based treatment recommendations available for SMECE (10). This has been limited by rarity of cases and thus the restricted knowledge of the disease. The most common treatment is surgical resection, with or without neck nodal dissection (10). Thyroid SMECE was initially described as a low-grade carcinoma (1). Adjuvant therapies include radiotherapy, chemotherapy and radioactive iodine ablation and hormone suppression post thyroidectomy (11). SMECE can usually be managed with surgical resection. For patients with aggressive disease or metastasis, more extensive treatment interventions are required (11).
SMECE of the thyroid gland is an uncommon but potentially aggressive cancer with no standard treatment that can be easily mistaken for other pathologies. On FNA sampling, differential diagnoses should be considered including SCC of the thyroid, anaplastic carcinoma, PTC, NS-HL, salivary MEC and Hashimoto’s thyroiditis. Depending on clinical status, the most common treatment option is surgical resection with or without lymph node resection. For more aggressive cases, positive margins or metastasis, adjuvant therapy should be considered.
Acknowledgments
We would like to acknowledge our patient who provided informed written consent for de-identified data to appear in this case report.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://aot.amegroups.com/article/view/10.21037/aot-21-17/rc
Peer Review File: Available at https://aot.amegroups.com/article/view/10.21037/aot-21-17/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://aot.amegroups.com/article/view/10.21037/aot-21-17/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan JK, Albores-Saavedra J, Battifora H, et al. Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto's thyroiditis. Am J Surg Pathol 1991;15:438-48. [Crossref] [PubMed]
- Lai CY, Chao TC, Lin JD, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of thyroid gland in a male patient: a case report and literature review. Int J Clin Exp Pathol 2015;8:5947-51. [PubMed]
- Chambers M, Nosé V, Sadow PM, et al. Salivary-Like Tumors of the Thyroid: A Comprehensive Review of Three Rare Carcinomas. Head Neck Pathol 2021;15:212-24. [Crossref] [PubMed]
- Baloch ZW, Solomon AC. LiVolsi VA. Primary mucoepidermoid carcinoma and sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: a report of nine cases. Mod Pathol 2000;13:802-7. [Crossref] [PubMed]
- Shah AA, La Fortune K, Miller C, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol 2017;30:329-39. [Crossref] [PubMed]
- Sukumar JS, Sukumar S, Purohit D, et al. Activating BRAF mutation in sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: two case reports and review of the literature. J Med Case Rep 2019;13:385. [Crossref] [PubMed]
- Pantola C, Kala S, Athar M, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: A cytological dilemma. J Cytol 2016;33:37-9. [Crossref] [PubMed]
- Solomon AC, Baloch ZW, Salhany KE, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: mimic of Hodgkin disease in nodal metastases. Arch Pathol Lab Med 2000;124:446-9. [Crossref] [PubMed]
- Pileri SA, Ascani S, Leoncini L, et al. Hodgkin's lymphoma: the pathologist's viewpoint. J Clin Pathol 2002;55:162-76. [Crossref] [PubMed]
- Orbeal R, Jimeno J, Monroy G, et al. Sclerosing mucoepidermoid carcinoma of the thyroid gland with eosinophilia. Cir Esp 2015;93:e137-8. [Crossref] [PubMed]
- Lee JH, Ayala AG, Ro JY. Commentary on sclerosing mucoepidermoid carcinoma with eosinophilia of thyroid glands. J Med Surg Pathol 2016; [Crossref]
Cite this article as: Heaven CL, Shetty S, de Beer JG. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: first Australasian case report. Ann Thyroid 2022;7:1.


