Narrative review of neck reinterventions for medullary thyroid carcinoma: indications and outcomes
Introduction
Medullary thyroid carcinoma (MTC) is a rare and malignant neuroendocrine tumor arising from the parafollicular C-cells of the thyroid gland, and it represents 1–2% of all thyroid malignancies. It can be either sporadic (75%) or hereditary (25%), the latter associated with type 2 multiple neuroendocrine neoplasia syndromes (MEN2A and MEN2B) or familial MTC. C-cells secrete the hormone calcitonin (CTN). Serum CTN is elevated in virtually all cases, and it is of major importance in the diagnosis and follow-up of MTC patients (1,2). In doubtful cases, a CTN stimulation test might be necessary to confirm the diagnosis. Nevertheless, elevated serum CTN levels is not an exclusive characteristic of MTC and may be present in other malignancies (neuroendocrine tumors, lung cancer, etc.); the measurement of CTN levels in the fine needle aspiration biopsy (FNAB) aspirate helps confirming the diagnosis of MTC. Although MTC has a worse prognosis than differentiated thyroid carcinoma, it still has an acceptable 10-year overall survival rate (3,4). In a recent study by Kotwal et al. (5) in 163 MTC patients, with a median follow-up of 5 years, the overall survival rates were 89.8% and 81.2% at 5 and 10 years, respectively. As regards distant metastases, they are the main cause of death related to MTC, with a survival rate of approximately 25% and 10% at 5 and 10 years, respectively, from their diagnosis (6).
Unlike differentiated thyroid carcinoma, where follicular thyroid cells respond to radioactive iodine, MTC C-cells are not sensitive to it, thus making surgical approach of great importance to achieve disease control. Preoperative assessment is essential in order to determine the extent of the disease and design the surgical strategy. The Revised American Thyroid Association (ATA) Guidelines for the Management of MTC (1) state that the initial surgical strategy for patients with diagnosed MTC (thyroid nodule with FNAB diagnostic for MTC and elevated serum CTN levels), both sporadic or hereditary, without evidence of cervical lymph node metastases in the lateral compartments and no evidence of distant metastases is total thyroidectomy with bilateral central compartment (level VI) lymph node dissection. Whenever macroscopic cervical lymph node metastases are present at diagnosis in the lateral lymph node compartments (levels II–V), they must be removed following a compartment-oriented strategy during the initial surgery. Given the high number of patients with MTC that develop cervical lymph node metastases during follow-up, and the risks associated with repeated neck operations, there is concern on whether cervical lateral compartment lymph node dissection should be carried out during the initial surgery in patients without evidence of cervical lymph node metastases on ultrasound (US)-examination. Most authors agree that preoperative serum CTN levels should guide the decision whether to proceed with lateral compartment lymph node dissection in the absence of macroscopic lymph node metastases. There is no consensus on how to proceed in patients with normal neck US: while some endocrinologists and surgeons believe that preoperative neck US should guide the decision and do not advocate lateral compartment lymph node dissection in negative preoperative neck US, others consider it indicated if basal serum CTN levels are between 20 and 50 pg/mL. Moreover, some authors believe that cervical contralateral lateral compartment lymph node dissection is indicated in patients with a cervical negative US when basal serum CTN levels are >200 pg/mL (1,7-9).
Approximately 70% of patients with palpable MTC present nodal cervical metastases at diagnosis, which has a negative impact on prognosis (10-12). Moreover, up to 50% of patients with presumed curative surgery have persistent postoperative elevated serum CTN levels, in most of the cases due to inadvertent residual malignant tissue or metastases—mostly in the cervical region—and 10–25% develop cervical recurrence that may compromise vital structures such as trachea or esophagus (7).
No medical curative treatment has proven effective in the treatment of locally advanced or metastatic MTC, being surgery the only curative option. Chemotherapy and external beam radiation have limited response rates. Vandetanib and cabozantinib are the only tyrosine kinase inhibitors (TKIs) approved for the treatment of locally advanced and metastatic MTC non-amenable to surgery, having demonstrated an improved progression-free survival but without an impact on overall survival. Immunotherapy with PD-L1 inhibitors (pembrolizumab), or peptide receptor radionuclide therapy (PRRT) are other therapeutic options that are being evaluated and might prove effective in selected cases (13).
Given the actual controversy regarding the surgical treatment of locally advanced and metastatic MTC, we have conducted this narrative review to discuss the cervical recurrence risk factors of MTC and the indications, recommended surgical strategy and outcomes of cervical reintervention.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/aot-21-12).
Methods
We searched in PubMed and Web of Science all articles on MTC surgery published in English between 1990–2021. An additional secondary review of the bibliography references of the selected articles was performed.
Postoperative initial evaluation: has biochemical cure been achieved?
Normalization of serum CTN levels after optimal surgery, together with disease burden at diagnosis, are considered the most important prognostic factors in patients with MTC that have undergone surgery with a curative intent (5,14). Undetectable serum CTN levels after surgery can be considered a curation criterion and implies a low risk of recurrence (15). There is no consensus as regards the time needed for serum CTN levels to reach its nadir. Based on the recommendations from the ATA, serum CTN and carcinoembryonic antigen (CEA) levels must be measured 3 months postoperatively in order to determine if the initial surgery has been curative, considering biochemical cure if serum CTN levels are undetectable or within the normal range (<10 pg/mL). If this is the case, serum CTN and CEA levels should be measured every 6 months during the first year, and once a year thereafter (1,5,15). In the case of biochemical cure after surgery, imaging techniques seem unnecessary while serum CTN and CEA levels remain within the normal range. Machens et al. (16) analyzed 474 MTC patients treated with total thyroidectomy and compartment-oriented neck dissection. Among patients with biochemical cure after surgery, no recurrences were observed during follow-up in the neck compartments that were dissected during initial surgery (mean follow-up of 61.1, 45.5 and 49.7 months for central, ipsilateral lateral and contralateral lateral neck compartments, respectively). This leads the authors to conclude that follow-up with serum CTN levels measurements alone in patients with postoperative biochemical response is adequate, and imaging techniques should be reserved for patients without biochemical cure or when serum CTN levels start to increase after initial normalization.
Persistence or recurrence after initial surgery: when, how and why
Difference between persistence and recurrence after initial surgery in the setting of MTC is not clearly established, and many of the articles published on this subject refer to both terms indistinctively. Both persistence and recurrence can be only biochemical (elevated serum CTN levels without evidence of clinical disease) or structural (imaging evidence of organic lesions).
Structural recurrence rates vary from 10% to 27%, with a median time until histology-proven local recurrence of 4 years, with a wide temporal range that can go from 8 to 156 months in the published series. Machens et al. (16) report an overall recurrence, node recurrence and soft tissue infiltrate recurrence of 45.5, 34.0 and 49.4 months, respectively, in the ipsilateral lateral neck; 49.7, 49.7 and 76 months, respectively, in the contralateral lateral neck; and 61.1, 63.6 and 80.8 months, respectively, in the central neck. Kuo et al. (7) reported a reintervention rate of 18.6% in an observational retrospective study from the data of the California Cancer Registry, with a median time to reintervention of 6 months. On the contrary, Verbeek et al. (17) reported a median time to reintervention of 33 [4–331] months. This variability could be probably explained by the above-mentioned fact that there is not a clear differentiation between persistence/recurrence and biochemical/structural disease. It is possible that the groups that report shorter intervals until reintervention included a higher proportion of reinterventions due to biochemical persistence or completion surgeries for an initial sub-optimal surgery, while the ones that report longer intervals are reoperating histology-proven structural disease in a higher proportion.
Nodal disease is the main form of recurrence (16). Tumor size, extrathyroidal extension and number of affected lymph nodes excised during initial surgery are the main risk factors for local recurrence. Verbeek et al. (17) reported TNM stage ≥ IVa and lymph node involvement were significant predicting factors for clinical recurrence. Kotwal et al. (5) reported a local recurrence rate of 9.8%, but only two patients among those with local recurrence (16 patients) and distant metastases (25 patients) reached biochemical cure after initial surgery, thus suggesting that in most cases there was persistence rather than recurrence. In their study, male sex, nodal status ≥N1b, ≥M1 (both according to AJCC), gross extrathyroidal extension, tumor size, ≥5 lymph nodes affected, ratio of involved/resected lymph nodes and higher post-operative serum CTN levels were significant predictive factors for local recurrence on univariate analysis, but only gross extrathyroidal extension, ≥ N1b status and involved/resected lymph node ratio remained significant risk factors for local recurrence in multivariate analysis. Machens et al. (16) analyzed the recurrence pattern of 474 patients with MTC treated with total thyroidectomy with central (100%) and lateral (80.6%) lymph node dissection. They observed that tumor size >20 mm and extranodal growth was associated with nodal recurrence both in central and ipsilateral lateral neck, but not in the contralateral lateral neck, where the incidence was so low that no statistical analysis was performed. Local recurrence in which no nodal parenchyma was seen in the surgical specimen was considered as soft tissue infiltrate recurrence. The risks factors for locoregional soft tissue infiltrate recurrences were extrathyroidal extension when they appeared in the central neck [odds ratio (OR) 20.2], and extranodal growth when in the ipsilateral lateral compartments (OR 6.4), suggesting that tumoral and nodal capsule breaching may represent an important source of local recurrence. Anecdotally, local recurrence associated with percutaneous ablation procedures has also been reported (18).
Apart from patient/tumor characteristics, we must also highlight the importance of an optimal initial surgery to reduce the risks of persistence/recurrence. Verbeek et al. (17) compared the results of MTC patients operated in two tertiary referral centers in the Netherlands with regard to the adherence to ATA guidelines recommendations, and concluded that compliance with those guidelines resulted in fewer reinterventions for local persistence/recurrence and a higher rate of biochemical cure, thus emphasizing that an optimal initial surgery has a great impact on prognosis.
Indications for neck reintervention
Neck reintervention implies greater risks in term of recurrent laryngeal nerve injury, thoracic duct leak and hypoparathyroidism, most of them permanent and with great impact on quality of life, so the decision to reoperate should be carefully taken (8,19). The probability of achieving biochemical cure after neck reintervention should guide the decision, searching a balance between surgical risks and benefits (20). In these circumstances, surgical and histopathological reports of previous interventions are of major importance before reintervention, and a preoperative evaluation of the vocal cords through laryngoscopy is considered necessary (12).
Indications for neck reintervention in patients with sub-optimal initial surgery
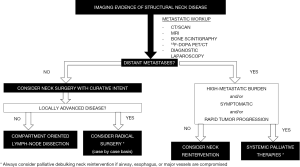
In those situations when MTC is diagnosed after a thyroidectomy performed for another reason, there is no consensus on whether to proceed with reoperation in order to perform an optimal oncological surgery or rather adopt a watchful-waiting strategy. Given the probability of multifocal and bilateral disease in hereditary MTC, most authors agree that completion thyroidectomy should be performed in these cases after an initial lobectomy. As regards sporadic MTC, there is no consensus and the decision to reoperate should be based on postoperative serum CTN levels and the presence of detectable disease confined to the neck. Therefore, the decision to proceed with reoperation of patients diagnosed with MTC should be reserved for patients that have not achieved biochemical cure after initial suboptimal surgery, patients with RET germline mutations, or if there is imaging evidence of recurrent or persistent local disease (1,8) (Figure 1).
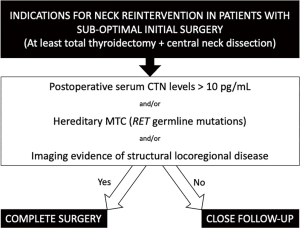
On the other hand, when no risk factors are present and biochemical cure is achieved, a watch-and-wait approach could be enough. This is reflected in the results published by Kuo et al. (7), who analyzed 609 patients who underwent thyroid surgery for MTC. Forty-six patients underwent thyroid lobectomy during initial surgery, not followed by completion thyroidectomy with central compartment lymphadenectomy as recommended in the ATA guidelines, and only 1 patient (2.2%) had neck reintervention during follow-up.
Indications for neck reintervention in patients without biochemical cure after presumed optimal initial surgery
In up to 50% of cases where surgery was presumed to be optimal biochemical cure is not achieved, and failure to normalize serum CTN levels indicates the presence of residual or recurrent disease (7,8). Whenever there is evidence of persistent/recurrent disease confined to the neck, reintervention should be considered. However, not all patients with persistent elevated serum CTN levels after adequate initial surgery have detectable persistent or recurrent disease. In these cases, there is no evidence of the significance of this elevation and survival may not me impaired, but at least a close follow up is advised, because while many patients will remain without clinical recurrence, others with more aggressive forms of MTC may develop a rapidly progressive disease with significant impact on survival (8,14,19).
Follow-up, as recommended by the ATA Guidelines (1), differs depending on postoperative serum CTN levels, which together with serum CEA levels must be measured every 6 months in every case in order to determine their doubling time, which reflects the MTC growth and progression rates: (I) postoperative serum CTN detectable levels but <150 pg/mL indicate residual or recurrent disease confined to the neck, so physical examination and US of the neck must be performed and, if negative, they should be repeated every 6 months; (II) postoperative serum CTN levels >150 pg/mL indicate high probability of distant metastases, so whole-body imaging examinations should be performed in order to rule them out, which may include neck US, chest and abdominal computed tomography (CT)-scan, bone scintigraphy and magnetic resonance imaging (MRI) of the pelvis and axial skeleton. The use of other techniques, such as diagnostic laparoscopy or positron emission tomography (PET)/CT will be further discussed.
Indications for surgery in patients with structural disease
In most cases, recurrent/persistent disease in the neck and mediastinum can be surgically removed either with a curative or a palliative intent (6). In the absence of metastatic spread, surgery should be considered as a first-line curative treatment. On the contrary, in case of locally advanced disease or metastatic spread, surgery may not be planned with a curative intent but rather as a palliative procedure with the aim of minimizing complications derived from invasion of vital structures or organs (12) (Figure 2).
Patients with structural neck disease and with a low-metastatic burden, with no evidence of metastatic progression and without extracervical-related symptoms should be considered for neck reintervention, whereas in those cases in which extracervical disease is rapidly progressing or symptomatic, systemic palliative therapies might be the best option (6). There is controversy on the role of neck reinterventions in biochemical persistent disease in the presence of extracervical metastases. Some groups consider that the long lifespan of MTC patients with distant metastases justifies radical neck reinterventions (20), while others suggest that they should be avoided (21).
Even under these circumstances, palliative surgery for structural recurrence that threatens vital organs should always be contemplated in order to prevent compression and/or invasion of cervical vital structures such as trachea, esophagus or major vessels. In a classic paper by Moley et al. (21), they report 7 palliative reoperations for locally advanced MTC, with 6 patients alive at the end of the follow-up period, ranging from 6 to 25 months. Of these 6 patients, 5 did not develop bulky cervical recurrence nor had their airway or digestive tract compromised during follow-up, and 1 patient died 14 months after surgery due to progression of metastatic disease.
The risks and potential benefits of neck reintervention in locally advanced disease, either curative or palliative, should be carefully assessed in a case-by-case basis as quite mutilating procedures might be necessary (18). Unfortunately, extensive nodal recurrence and/or metastatic spread are quite common in these circumstances, and only 20% of locoregional invasive recurrences are candidates for radical surgery (22).
Metastatic workup before neck reintervention
Preoperative assessment is of key importance in MTC patients in order to determine the extent of the disease before neck reintervention. As stated before, the ATA Guidelines (1) recommend US-neck examination, chest and abdominal CT-scan, bone scintigraphy and MRI of the pelvis and axial skeleton, depending on serum CTN levels. Imaging techniques have a considerable high rate of false negative results as regards the localization of metastatic foci when serum CTN levels are <150 pg/mL. The sensitivity of imaging techniques in recognizing distant metastasis is higher when serum CTN levels are >150 pg/mL (PET/CT) and >500–1,000 pg/mL (CT, MRI). In every case, liver micrometastasis should always be suspected. Exploratory laparoscopy was recommended by Tung et al. (23) in order to rule out superficial liver metastases that might not be evident with other imaging techniques (mainly CT and MRI scans). In a series of 41 patients, they found 8 patients with liver metastases, of whom 7 had normal CT or MRI scans. However, the rapid development of imaging techniques in the past decades obliges us to reconsider the pertinence of using invasive techniques such as exploratory laparoscopy, but more studies are needed to determine which is the preferred technique for this purpose. Although the ATA Guidelines (1) state that “neither FDG-PET/CT nor F-DOPA-PET/CT is recommended to detect the presence of distant metastases. Grade E Recommendation”, there is growing evidence that nuclear medicine imaging techniques such as PET/CT represent a promising alternative. Although there is not yet consensus on which PET radiopharmaceutical shows the best detection rate of structural disease (either persistence/recurrence or metastatic disease) in MTC patients with postoperative elevated serum CTN levels, it seems that, with the evidence available to date, 18F-dihydroxyphenylalanine (18F-DOPA) has the best performance (24,25). Terroir et al. (24) studied the detection rates of different imaging techniques (18F-DOPA PET/CT, 18F-FDG PET/CT, whole body MRI and CT scans, neck-US and bone scintigraphy) in MTC patients with elevated postoperative serum CTN levels. 18F-DOPA PET/CT showed a better detection rate of locoregional or disseminated disease than any other imaging technique, being its sensitivity higher when serum CTN levels were >150 pg/mL (90%), in contrast to <150 pg/mL (30%). A recent meta-analysis by Lee et al. (25) compared 5 different PET radiopharmaceuticals (18F-FDG, 18F-DOPA, 68Ga-somatostatin analogs, 3-O-methyl-6-[18F]fluoro-DOPA, and 11C-methionine) in the detection of recurrent disease in MTC patients. The results showed that 18F-DOPA had the best performance in detecting recurrent disease, regardless of serum CTN and CEA levels. The European Association of Nuclear Medicine has recently published practice guidelines for PET/CT imaging in MTC (26). They recommend the use of PET/CT scanning for the evaluation of MTC patients with postoperative elevated or rising tumor markers, mainly when serum CTN levels >150 pg/mL or with shortened doubling times. 18F-DOPA PET/CT, if available, should be the first choice because of the better performance of 18F-DOPA as compared to other radiopharmaceuticals; if negative or unavailable, 18F-FDG PET/CT should be considered, especially if tumor markers are rapidly rising or an aggressive behavior of the disease is expected.
Surgical procedures and outcomes of neck reintervention in structural recurrence/persistence
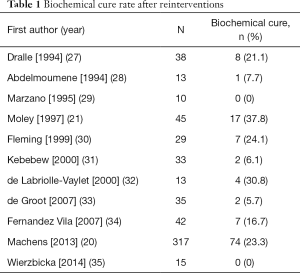
If neck reintervention is indicated with a curative intent, the goal should be to achieve biochemical cure. The reported outcomes after successful initial surgery and after reoperation to complete a sub-optimal initial surgery are similar in terms of reoperations and biochemical cure at last follow-up, provided that serum CTN levels are normalized after reintervention (17). This means that there can be a “second-chance” for these patients even if the initial surgery was not curative. This finding could be especially relevant in patients that were initially treated without a preoperative suspicion of MTC and those initially treated in low-volume centers. However, reintervention does not assure biochemical cure, and the rate of postoperative serum CTN normalization reported in the literature ranges from 0% to 40% (Table 1). Rowland et al. (36) published a meta-analysis (27 studies, 984 patients) on the rate of biochemical cure after reoperation for MTC, which was 16.2% (14.0–18.5%).
Full table
A balance between surgical risks and survival benefits must guide our decision whether to proceed with reintervention or not, and any factor that could predict the reintervention outcomes should be taken into consideration. Postoperative serum CTN levels and the number of metastatic lymph nodes removed during initial surgery seem to be the best predictors of the probability of achieving biochemical cure after reintervention. According to Machens and Dralle (20), if serum CTN levels are <1,000 pg/mL and/or there were <5 metastatic lymph nodes removed, biochemical cure rates after reintervention can vary between 18–44% with an acceptable morbidity, and therefore reintervention could be worthwhile in experience hands. However, biochemical cure rates after reintervention drop to 1–5% if serum CTN levels are >1,000 pg/mL and/or there were >5 metastatic lymph nodes removed, so the balance between surgical risks and benefits in this setting is not favorable. In these cases, extensive neck dissections with a curative intent might not be indicated and surgery may be reserved for those situations when vital structures such as major vessels, trachea or esophagus are compromised due to structural disease (12,20).
There is no consensus on the extent of the dissection that is necessary to achieve biochemical cure. The meticulous removal of all lymph nodes and fatty tissue in the neck (all compartments in the central and lateral neck, from the mastoid process to the innominate vein and subclavian arteries, sometimes even including upper mediastinal lymph nodes) was first published by Tisell et al. in 1986 (37) and termed “microdissection”, with good results in terms of achieving biochemical cure that has been reproduced by other groups (8,19). Although the extent of the dissection as published by Tisell et al. may be unnecessary in some cases, there is consensus that a compartment-oriented lymph-node dissection is the recommended surgical strategy as it has demonstrated to improve recurrence and survival rates as compared to a limited lymphadenectomy or “berry picking”, which could be reserved for palliative situations (8,10,16,20,38,39). In the previously mentioned meta-analysis by Rowland et al. (36), the rate of serum CTN normalization was higher when a compartment-oriented procedure was performed (18.6%; 95% CI: 15.9–21.3%) as compared to a limited lymphadenectomy with selective lymph node removal (10.5%; 95% CI: 6.4–14.7%).
Fialkowski et al. (19) published one of the largest series of reoperated MTC patients with a long-term follow-up, reporting the postoperative outcomes of 50 patients. According to serum CTN levels 8–10 years after reoperation, 13 (26%) patients had lower serum CTN levels than those before reoperation, and 14 (28%) patients had biochemical cure at the end of follow-up. According to these results, the authors conclude that long-term eradication of MTC is possible even after reoperation. The results reported by Kuo et al. (7) from the California Cancer Registry, with a median follow-up of 7.7 years, show that prognosis in terms of survival is not impaired in reoperated MTC patients, which leads the authors to conclude that reoperation benefits could outweigh the inherent risks of recurrence.
Complications after neck reintervention
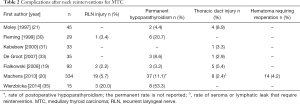
Neck reintervention is associated with a higher risk of complications than during primary surgery, reaching as much as 8% for recurrent laryngeal nerve injury, 16–25% for permanent hypoparathyroidism and 8% for thoracic duct leak, and the risk is bigger as more nodal metastases are removed (14,39). It is important to note that most of the articles reporting complications after neck reinterventions for MTC are published by the two groups with the largest series of MTC patients (19,20), so the good results reported might be strongly influenced by their wide experience. Indeed, other groups with smaller series report higher complication rates (35) (Table 2).
Full table
Conclusions
Surgery is the cornerstone of the treatment of MTC patients. Reintervention is indicated in cases of locoregional recurrence, both nodal or soft tissue infiltrates, in order to achieve biochemical cure, as the benefits of removing all structural disease could outweigh the risks of neck reintervention. In these cases, overall survival is still good, provided biochemical cure is achieved. Palliative neck reintervention should be considered whenever vital structures are compromised.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/aot-21-12
Peer Review File: Available at http://dx.doi.org/10.21037/aot-21-12
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-21-12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
- Frank-Raue K, Machens A, Leidig-Bruckner G, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid 2013;23:294-300. [Crossref] [PubMed]
- Kebebew E, Ituarte PH, Siperstein AE, et al. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139-48. [Crossref] [PubMed]
- Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’étude des tumeurs à calcitonine. Clin Endocrinol (Oxf) 1998;48:265-73. [Crossref] [PubMed]
- Kotwal A, Erickson D, Geske JR, et al. Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma over Two Decades. Thyroid 2021;31:616-26. [Crossref] [PubMed]
- Schlumberger M, Bastholt L, Dralle H, et al. 2012 European thyroid association guidelines for metastatic medullary thyroid cancer. Eur thyroid J 2012;1:5-14. [Crossref] [PubMed]
- Kuo EJ, Sho S, Li N, et al. Risk factors associated with reoperation and disease-specific mortality in patients with medullary thyroid carcinoma. JAMA Surgery 2018;153:52-9. [Crossref] [PubMed]
- Jin LX, Moley JF. Surgery for lymph node metastases of medullary thyroid carcinoma: A review. Cancer 2016;122:358-66. [Crossref] [PubMed]
- Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2655-63. [Crossref] [PubMed]
- Moley JF. Medullary thyroid carcinoma: management of lymph node metastases. J Natl Compr Canc Netw 2010;8:549-56. [Crossref] [PubMed]
- Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: recommendations for extent of node dissection. Ann Surg 1999;229:880-7; discussion 887-8. [Crossref] [PubMed]
- Konstantinidis A, Stang M, Roman SA, et al. Surgical management of medullary thyroid carcinoma. Updates Surg 2017;69:151-60. [Crossref] [PubMed]
- Grande E, Santamaría Sandi J, Capdevila J, et al. Consensus on management of advanced medullary thyroid carcinoma on behalf of the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) and the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI). Clin Transl Oncol 2016;18:769-75. [Crossref] [PubMed]
- Gimm O, Ukkat J, Dralle H. Determinative factors of biochemical cure after primary and reoperative surgery for sporadic medullary thyroid carcinoma. World J Surg 1998;22:562-7; discussion 567-8. [Crossref] [PubMed]
- Viola D, Elisei R. Management of Medullary Thyroid Cancer. Endocrinol Metab Clin North Am 2019;48:285-301. [Crossref] [PubMed]
- Machens A, Lorenz K, Dralle H. Histology-proven recurrence in the lateral or central neck after systematic neck dissection for medullary thyroid cancer. Endocrine 2018;61:428-39. [Crossref] [PubMed]
- Verbeek HHG, Meijer JAA, Zandee WT, et al. Fewer Cancer Reoperations for Medullary Thyroid Cancer After Initial Surgery According to ATA Guidelines. Ann Surg Oncol 2015;22:1207-13. [Crossref] [PubMed]
- Muñoz de Nova JL, Dworzynska A, Lorente-Poch L, et al. Esophageal recurrence of medullary thyroid carcinoma. Gland Surgery 2015;4:564-6. [PubMed]
- Fialkowski E, Debenedetti M, Moley J. Long-term outcome of reoperations for medullary thyroid carcinoma. World J Surg 2008;32:754-65. [Crossref] [PubMed]
- Machens A, Dralle H. Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg 2013;257:751-7. [Crossref] [PubMed]
- Moley JF, Dilley WG, Debenedetti MK. Improved results of cervical reoperation for medullary thyroid carcinoma. Ann Surg 1997;225:734-40; discussion 740-3. [Crossref] [PubMed]
- Machens A, Hinze R, Dralle H. Surgery on the cervicovisceral axis for invasive thyroid cancer. Langenbecks Arch Surg 2001;386:318-23. [Crossref] [PubMed]
- Tung WS, Vesely TM, Moley JF. Laparoscopic detection of hepatic metastases in patients with residual or recurrent medullary thyroid cancer. Surgery 1995;118:1024-9; discussion 1029-30. [Crossref] [PubMed]
- Terroir M, Caramella C, Borget I, et al. F-18-Dopa Positron Emission Tomography/Computed Tomography Is More Sensitive Than Whole-Body Magnetic Resonance Imaging for the Localization of Persistent/Recurrent Disease of Medullary Thyroid Cancer Patients. Thyroid 2019;29:1457-64. [Crossref] [PubMed]
- Lee SW, Shim SR, Jeong SY, et al. Comparison of 5 Different PET Radiopharmaceuticals for the Detection of Recurrent Medullary Thyroid Carcinoma: A Network Meta-analysis. Clin Nucl Med 2020;45:341-8. [Crossref] [PubMed]
- Giovanella L, Treglia G, Iakovou I, et al. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging 2020;47:61-77. [Crossref] [PubMed]
- Dralle H, Damm I, Scheumann GF, et al. Compartment-oriented microdissection of regional lymph nodes in medullary thyroid carcinoma. Surg Today 1994;24:112-21. [Crossref] [PubMed]
- Abdelmoumene N, Schlumbergerl M, Gardet P, et al. Selective venous sampling catheterisation for localisation of persisting medullary thyroid carcinoma. Br J Cancer 1994;69:1141-4. [Crossref] [PubMed]
- Marzano LA, Porcelli A, Biondi B, et al. Surgical management and follow‐up of medullary thyroid carcinoma. J Surg Oncol 1995;59:162-8. [Crossref] [PubMed]
- Fleming JB, Lee JE, Bouvet M, et al. Surgical Strategy for the Treatment of Medullary Thyroid Carcinoma. Ann Surg 1999;230:697-707. [Crossref] [PubMed]
- Kebebew E, Kikuchi S, Duh QY, et al. Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg 2000;135:895-901. [Crossref] [PubMed]
- de Labriolle-Vaylet C, Cattan P, Sarfati E, et al. Successful surgical removal of occult metastases of medullary thyroid carcinoma recurrences with the help of immunoscintigraphy and radioimmunoguided surgery. Clin Cancer Res 2000;6:363-71. [PubMed]
- de Groot JW, Links TP, Sluiter WJ, et al. Locoregional control in patients with palpable medullary thyroid cancer: Results of standardized compartment-oriented surgery. Head Neck 2007;29:857-63. [Crossref] [PubMed]
- Fernandez Vila JM, Peix JL, Mandry AC, et al. Biochemical results of reoperations for medullary thyroid carcinoma. Laryngoscope 2007;117:886-9. [Crossref] [PubMed]
- Wierzbicka M, Gurgul E, Wasniewska-Okupniak E, et al. The feasibility and efficacy of secondary neck dissections in thyroid cancer metastases. Eur Arch Otorhinolaryngol 2014;271:795-9. [Crossref] [PubMed]
- Rowland KJ, Jin LX, Moley JF. Biochemical Cure after Reoperations for Medullary Thyroid Carcinoma: A Meta-analysis. Ann Surg Oncol 2015;22:96-102. [Crossref] [PubMed]
- Tisell LE, Hansson G, Jansson S, et al. Reoperation in the treatment of asymptomatic metastasizing medullary thyroid carcinoma. Surgery 1986;99:60-6. [PubMed]
- Welch K, McHenry CR. Selective lateral compartment neck dissection for thyroid cancer. J Surg Res 2013;184:193-9. [Crossref] [PubMed]
- Yen TWF, Shapiro SE, Gagel RF, et al. Medullary thyroid carcinoma: results of a standardized surgical approach in a contemporary series of 80 consecutive patients. Surgery 2003;134:890-9. [Crossref] [PubMed]
Cite this article as: Muñoz de Nova JL, Valdés de Anca Á, Torres Mínguez E, Martín-Pérez E. Narrative review of neck reinterventions for medullary thyroid carcinoma: indications and outcomes. Ann Thyroid 2021;6:11.