
Isolated medullary thyroid carcinoma: a case report
Introduction
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor, driven from parafollicular C-cell origin, which occurs in two forms; sporadic and familial. MTC encompasses about 3% of all thyroid cancers within the United States (1,2). Those of hereditary provenance concerning the RET proto-oncogene account for about 25% of MTC diagnosis’, while the remaining 75% are believed to be of the sporadic form (2). Familial forms are inherited via autosomal dominance with germline mutations on chromosome 10q11 (2). This form of MTC is also associated with other endocrine tumors in syndromes known as multiple endocrine neoplasia (MEN) 2A and MEN 2B (3). Those with MEN 2A, in addition to MTC, have a greater risk of developing pheochromocytoma and parathyroid adenomas. MEN2B, on the other hand, includes MTC, mucosal neuromas, pheochromocytomas, and marfanoid body habitus (3). Preventive thyroidectomy may be considered in patients expressing RET proto oncogene mutation. However, ethical issues may arise where, in the case of hereditary form, relatives may refuse to disclose their condition to family members, or parents may refuse to allow thyroidectomy to their children (4).
Some of the distinguishing features of MTC in contrast to other forms of thyroid cancer include female predominance, regional lymph node metastasis followed by subsequent spread to distant organs such as the liver, brain, bone, and adrenal medulla (5,6). Additionally, MTCs have no linked association with radiation exposure and have been found to originate within the upper central lobe of the thyroid gland (6). Other differential diseases such as hyper/hypothyroidism, toxic goiters, and lymphomas are ruled out with the use of TSH levels, radionucleotide uptake, and ultrasound findings.
The disease spreads from parafollicular, or also known as c-cell hyperplasia, resulting in elevated calcitonin levels to microscopically invasive carcinoma and eventually to grossly evident carcinoma (7). MTC has other forms of biochemical markers such as plasma carcinoembryonic antigen (CEA), serotonin, chromogranin A, and tryptase, but clinically calcitonin has been known to be far superior for diagnosis, maintenance, and prognosis of the disease. Prognosis of the disease is subordinate to several factors including but not limited to the patient’s confounding medical history, the extent of metastasis, diagnostic lead time, as well as modality of treatment and/or possible recurrence. Sporadic MTC commonly arises within the fourth or fifth decades of life. Although early diagnosis of sporadic MTC remains a challenge, calcitonin is the mainstay of the diagnostic evaluation being highly sensitive but having low specificity (8). It is critical to note that although widely used, calcitonin is not pathognomonic for MTC. Other diseases that display elevated levels of calcitonin include renal insufficiency, hypercalcemia, hypergastrinemia, and neuroendocrine tumors.
Total thyroidectomy is still considered to be the modality of treatment for both hereditary and sporadic MTC at this time (9). Differential diagnosis for spontaneous MTC may include neck inflammation due to cervical adenitis from infections, sarcoidosis, or benign masses like lipomas or hemangiomas. Calcitonin serum levels in RET mutations help isolate MTC from other causes of a neck mass. Most times the diagnosis of sporadic MTC is delayed and detected when a prominent thyroid nodule is detected on ultrasound or by palpation. At this point, it is believed to have already cervically metastasized (8). We present a case of sporadic MTC in a 46-year-old female patient with a similar presentation, with no association to Multiple Endocrine Neoplasia’s. Written consent was obtained by the patient to present the case. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aot-21-4).
Case presentation
A 46-year-old Caucasian female presented to the clinic due to a concern over localized swelling on the lateral aspect of her neck that started a couple of weeks ago and had progressively worsened. The patient was unable to recall any trigging events to the swelling. She denied taking any new medications and reported nothing improved the swelling. At the time of presentation, she denied any pain, allergies other than sulfa medications, difficulties swallowing, breathing, or speaking clearly.
History
The patient has ongoing history of type 2 Diabetes Mellitus controlled with Metformin, and history of nicotine use for 32 years.
Family history
Significant for hypertrophic obstructive cardiomyopathy (father and brother), diabetes mellitus, end stage renal disease (mother), and stroke (sister).
Physical exam
At presentation, the patient’s vital signs were stable. Upon physical exam, patient was noted to be alert, oriented, and anxious. Pupils were equal, round, and reactive to light with no noted pallor or conjunctival injection. Mucous membranes appeared to be moist, and bilateral tympanic membranes were normal with no effusion or fluid. Upon palpation of the neck, there was edema noted on the right anterolateral-aspect of the neck, as well as a palpable asymmetrically enlarged nodule on the right thyroid that was firm with no flatulence or surrounding erythema.
There was mild tracheal deviation noted to the left. Cardiovascular examination findings were unremarkable. The lungs were clear to auscultation with no wheezing, or signs of respiratory distress. The skin was warm and dry to touch with no rash, erythema, or urticaria noted. The remainder of the physical exam findings were unremarkable.
Diagnostic tests
High-resolution ultrasound of the thyroid revealed multiple inhomogeneous echogenic thyroid nodules consistent with a multinodular goiter.
High-resolution ultrasound findings disclosed within the right lobe, a 5.2×3.9×1.7 cm3 inhomogeneous echogenecity. A large solid mass in the upper mole measuring 2.5×1.7×1.82 cm3, as well an another large solid slightly inhomogeneous mass located at the middle and lower pole measuring 3.2×2.5×1.7 cm3. In the left lobe, there appeared to be a 5.9×1.2×1.3 cm3 inhomogeneous echogenicity with accompanying “tiny hypoechoic masses at the middle and lower pole” as well. The largest was said to be measuring about 3×3 mm2. There was also a 4×6×3 mm3 right isthmus nodule detected. The conclusion from the ultrasound findings determined there were multiple nodules, consistent with a multinodular goiter.
Thyroid nodule Fine Needle Aspiration displayed atypical cells, suspicious for malignancy.
Chest X-ray displayed tracheal deviation to the left of the midline (Figure 1).

Soft Tissue Neck CT scan revealed an enlarged thyroid gland with multiple lobulated nodules. Lymphadenopathy was noted in the right neck with imaging characteristics similar to the thyroid nodules. Given the presence of the thyroid gland and adjacent lymphadenopathy with characteristic thyroid tissue, neoplasm with lymph node metastasis is suspected and consistent with FNA. Enlarged thyroid and right neck lymphadenopathy caused tracheal shift and proximal trachea appeared to be narrowed along the posterior aspect due to thyroid enlargement. A 5 mm nodule also noted in the right upper lobe of the lung, which suggested metastasis (Figure 2).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committe(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained by the patient.
Treatment
The patient underwent a total thyroidectomy. This included both a right and left neck dissection along with central compartment dissection. Unfortunately, during the procedure the right recurrent laryngeal nerve was sacrificed. In the following months, the patient also underwent left central level 6 lymph node dissection.
Post operatively, the patient underwent 30 rounds of radiation to target any metastasis. She was started on Vitamin D supplements (2,000 units), Levothyroxine (137 mcg), and Calcitriol (0.25 mcg). Routine follow up involves close monitoring of her calcitonin levels along with CEA levels to detect any recurrence.
Following the loss of her right recurrent laryngeal nerve, the patient complained of dysphagia for both solids and liquids which prompted a videostroboscopy. The voice quality was noted to be severe, with irregular phase symmetry as well as incomplete glottic closure. Following the procedure, the patient opted to clinically observe her new onset hoarseness, dysphagia and vocal cord paralysis rather than undergo further management.
Discussion
Clinical presentation of those with MTC can manifest in many different forms. Most often, there is a palpable enlarged thyroid gland during physical examination. At this stage, it is believed that both hereditary and sporadic forms of MTC have metastasized to the cervical lymph nodes (1,2). Other manifestations include dysphagia, dyspnea, coughing, and hoarseness with the involvement of the recurrent laryngeal nerve (2). Those with elevated levels of calcitonin, a hormone secreted by the neuroendocrine parafollicular cells of the thyroid gland, also suffer from bouts of diarrhea. Hormones secreted by the tumor (calcitonin, prostaglandins, serotonin, or VIP) can induce gastrointestinal secretions and hypermotility resulting in diarrhea. Ectopic hormone release can also lead to Cushing’s syndrome and facial flushing.
The approach to diagnosing sporadic MTC is one that can be explored through various avenues. A thorough physical exam and history may direct you to unfamiliar hyperplasia which would propel one to undergo further testing, as was the case with our patient. The physician may start out by obtaining thyroid stimulating hormone (TSH) measurements. If the measurements of TSH return back as normal or high and are consistent with a solitary nodule, the next step would be to obtain an ultrasound of the neck. At this point, the radiologic assessment would determine the suspicion for malignancy, which would be guided by a fine needle aspiration biopsy, and clinical treatment would follow (6). Preoperative calcitonin screening has contributed to improved survival and cure rates according to a retrospective study done in 2019 between years 1980 and 2017. More MTC have been detected before advancing to later stages (10). Fine needle aspiration would depict the pathological features of MTC of a disarray of nesting pattern. It will encompass many cells within the fibrous stroma surrounded by large amounts of amyloid deposition. Diagnosis can further be confirmed through immunohistochemical stains of chromogranin, calcitonin, and CEA.
Treatment depends on the extent and severity of the disease. Patients with MTC who have no evidence of lymph node metastasis if treated early in the disease have a good prognosis, with low risk of recurrence. On the other hand, those with the nodal disease have a higher risk of recurrence as well as progression and persistence of the disease (2). Elevated levels of calcitonin have a significant trend with distant metastasis. Patients with lower elevations of calcitonin are able to undergo curative thyroidectomy without the fear of distant metastasis. Those who do suffer from metastasis will require more aggressive treatments with radiation. Dissection of cervical lymph node compartments, as well as total thyroidectomy secondary to total serum calcitonin levels and ultrasound findings, is considered to be the standard treatment for both hereditary and sporadic MTC patients (9). According to the Society of Surgical Oncology, ipsilateral neck dissection is only rendered in those patients suffering from sporadic MTC with palpable lateral neck lymph nodes or in those with involvement of the central neck compartment. A lot of literature has no preset notion on these guidelines but this may be because the practice of bilateral and central neck dissection has not been performed globally. Patients are advised to follow up every 3 months postoperatively with adequate monitoring of CEA levels and calcitonin levels which can be done annually. Radioactive iodine therapy has no role in treatment for MTC (6). This could be explained because the parafollicular cells of the thyroid that MTC originates from do not absorb iodine unlike other forms of thyroid cancer. Hence, iodine scanning could not be utilized in evaluation of distant metastasis of MTC to other organs as well (6).
Although patients may opt for total thyroidectomy, this does not always cure the disease. There is evidence explaining the recurrence of disease in many patients.
One of the most feared complications following both thyroid and parathyroid surgery are recurrent laryngeal nerve injuries (11). This injury induces a paresis or palsy of the vocal cord and may also lead to dysphagia as was the case with our patient. Thyroidectomy in a RET proto-oncogene positive with a normal functioning thyroid raises ethical concerns of maleficence as the surgery puts them at risk of this feared complications. Post surgical long term side effects, including lifelong adherence to medications is to be addressed as well as other surgical complications that may arise. Those suffering from symptomatic vocal cord paralysis that is recognized postoperatively now calls for injection augmentation. Vocal fold injection allows for placement of a filler within the lateral aspects of the thyroarytenoid/cricoarytenoid muscles which allows for any correction of glottal insufficiency leading to dysphonia, dysphagia, and hoarseness (12). Although this is an option for many, our patient opted out of receiving vocal cord injections.
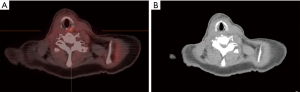
Post thyroidectomy, patients can expect to be placed on levothyroxine, a thyroid hormone analog, in order to maintain serum TSH levels within the euthyroid range (9). Routine PET/CT scans should be done to monitor for any suspicions or recurrence of disease (Figure 3). Patients should also continue to be monitored for hypocalcemia secondary to parathyroidectomy during surgery, which can be supplemented with calcium and calcitriol should symptoms arise.
Conclusions
Sporadic MTC accounts for 75% of all MTC s within the United States. Sporadic MTC can present at any age but is correlated with increased incidence within the fourth and fifth decades of life. Routine monitoring of serum calcitonin levels aids in detecting MTC and monitoring for recurrence. Surgical resection followed with radiation remains the standard treatment but comes with its own risks. Recurrent laryngeal nerve injury is common, and can be treated promptly following post thyroidectomy with vocal cord injections. Following total thyroidectomy, patients will need lifelong supplementation of thyroid hormone analog, vitamin D, and calcium.
Acknowledgments
Thank you to Dr. Cameron Shegos and Ron Faugue for their efforts in manuscript revision.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aot-21-4
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-21-4). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahmed SR, Ball DW. Clinical review: Incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab 2011;96:1237-45. [Crossref] [PubMed]
- Stamatakos M, Paraskeva P, Stefanaki C, et al. Medullary thyroid carcinoma: The third most common thyroid cancer reviewed. Oncol Lett 2011;2:49-53. [Crossref] [PubMed]
- Medullary Thyroid Cancer. American Thyroid Association. Accessed August 10, 2020. Available online: https://www.thyroid.org/medullary-thyroid-cancer/
- Rosenthal MS, Diekema DS. Pediatric ethics guidelines for hereditary medullary thyroid cancer. Int J Pediatr Endocrinol 2011;2011:847603 [Crossref] [PubMed]
- Smit J. Treatment of advanced Medullary Thyroid Cancer. Thyroid Res 2013;6 Suppl 1:S7. [Crossref] [PubMed]
- Clayman G. Medullary Thyroid Cancer: Overview. EndocrineWeb. Accessed September 18, 2020. Available online: https://www.endocrineweb.com/conditions/thyroid-cancer/thyroid-cancer-medullary-cancer
- Maxwell JE, Sherman SK. Medical management of metastatic medullary thyroid cancer. Cancer 2014;120:3287-301. [Crossref] [PubMed]
- Correia-Deur JE, Toledo RA, Imazawa AT, et al. Sporadic medullary thyroid carcinoma: clinical data from a university hospital. Clinics (Sao Paulo) 2009;64:379-86. [Crossref] [PubMed]
- Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
- Torresan F, Mian C, Cavedon E, et al. Cure and survival of sporadic medullary thyroid carcinoma following systematic preoperative calcitonin screening. Langenbecks Arch Surg 2019;404:411-9. [Crossref] [PubMed]
- Joliat GR, Guarnero V, Demartines N, et al. Recurrent laryngeal nerve injury after thyroid and parathyroid surgery: Incidence and postoperative evolution assessment. Medicine (Baltimore) 2017;96:e6674 [Crossref] [PubMed]
- Mallur PS, Rosen CA. Vocal fold injection: review of indications, techniques, and materials for augmentation. Clin Exp Otorhinolaryngol 2010;3:177-82. [Crossref] [PubMed]
Cite this article as: Chaudhry AF, Khosla K, Muriuki CW. Isolated medullary thyroid carcinoma: a case report. Ann Thyroid 2021;6:13.



