
Parathyroidectomy in a patient treated with denosumab: a case report
Introduction
Primary hyperparathyroidism (PHPT) is a relatively common endocrine disorder, affecting approximately 1 in 1,000 people, with a female-to-male ratio of 3–4:1 and increased prevalence in postmenopausal women (1,2). In 80% of patients, PHPT is due to a single benign parathyroid adenoma, with multiglandular disease, often four-gland parathyroid hyperplasia, seen in the remaining group (2). In postmenopausal women, a population already at increased risk of osteoporosis, PHPT can hasten bone loss and cause alterations to bone microarchitecture that may increase the risk of fractures (3-5).
Denosumab, a human monoclonal antibody to receptor activator of nuclear factor kappa-Β ligand (RANKL) sold under the trade name Prolia (Amgen Inc., Thousand Oaks, CA), was approved by the FDA in 2010 for treatment of osteoporosis in postmenopausal women at high risk of fracture and in 2018 for glucocorticoid-induced osteoporosis (6). Early clinical trials demonstrated an increase in bone mineral density (BMD) and a reduction in fractures with small risk of osteonecrosis of the jaw and atypical femur fractures (7-9).
We were unable to find any prior reports concerning parathyroidectomy in patients receiving denosumab. We report our experience of 3.5 gland parathyroidectomy in one such patient, hoping to underscore the potential impact denosumab may have in complicating interpretation of intraoperative parathyroid hormone (PTH) measurements. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/aot-20-64).
Case presentation
A 68-year-old Caucasian woman with Stage 3b chronic kidney disease (CKD) not on dialysis was referred by her endocrinologist for surgical management of PHPT. Her relevant medical comorbidities include type 2 diabetes mellitus, osteoporosis, and immune thrombocytopenic purpura on chronic glucocorticoids (40 mg prednisone per day). Her medications are also notable for cholecalciferol (Vitamin D3) 2,000 IU daily. On presentation, her uncorrected serum calcium was elevated to 10.6 mg/dL, 25-hydroxy vitamin D was normal at 34 ng/mL, and intact PTH was elevated to 209 pg/mL. As per recent guidelines, parathyroidectomy was recommended. In-office ultrasound did not show any evidence of enlarged parathyroid tissue. A SPECT-CT with Tc-99m sestamibi radiotracer showed findings suspicious for a left inferior parathyroid adenoma.
In the interim, exactly two weeks prior to surgery, she received a single dose (her first) of 60 mg/mL subcutaneous injection of denosumab for her osteoporosis. Subsequently but before surgery, the patient had albumin of 3.9 g/dL and uncorrected serum calcium of 8.5 mg/dL. Baseline preoperative PTH was 465 pg/mL and pre-incision ultrasound was significant for a 1 cm hypoechoic focus in the region of the left thyrothymic ligament. After making a transcervical incision in the expected location, this gland was readily identified intraoperatively and a 402 mg mass was removed. After an initial drop, intraoperative PTH plateaued at approximately 200 pg/mL beginning at the 5-minute mark and continuing into the 20-minute mark (Figure 1). The association between denosumab and elevated PTH was considered, and the remaining glands were electively explored. Both glands on the right, weighing 96 mg and 49 mg, and less than one-half of the left upper gland (total specimen weight 5 mg), were removed. Intraoperative PTH measured at 145 pg/mL before declining to 82 pg/mL at the new 15-minute timepoint (Figure 1). All specimens removed were confirmed to be parathyroid tissue on frozen pathology review.
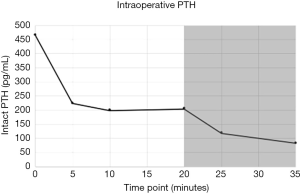
In the postoperative recovery room, PTH declined to 53 pg/mL, where it remained until discharge the next morning. Postoperatively, calcium was measured at 8.6 mg/dL, where it remained at time of discharge. At one-month follow up, PTH was slightly elevated at 87 pg/mL, although calcium was within normal limits at 9.1 mg/dL.
Ethics statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
As an antibody to RANKL, denosumab functions similarly to osteoprotegerin, competitively binding RANKL on osteoblasts and therefore preventing binding to receptor activator of nuclear factor kappa-Β (RANK), decreasing osteoclast formation and survival. In this way, denosumab is believed to exert an anabolic effect, preventing resorption of bone with consequent potential for extreme hypocalcemia and hyperparathyroidism. Dramatic elevations in PTH have been noted in several recent studies and case reports, even after just a single dose of denosumab (10-17).
Many of these prior reports suggest the risk of hypocalcemia and compensatory hyperparathyroidism is increased in patients with CKD (15-17). However, as with other antibodies, denosumab is believed to be metabolized by the reticuloendothelial system and not renally cleared (18). Furthermore, a study by Block et al. concluded renal function did not have a significant effect on denosumab pharmacokinetics or pharmacodynamics (19). In any case, PTH elevation may not occur in a predictable fashion, as considerable variability in PTH elevation has been described. Jang et al. described the course of two patients with ESRD on hemodialysis: both patients experienced asymptomatic hypocalcemia at a nadir of 6.8 mg/dL, 30 days after administration of denosumab. However, on day 30, patient 1 had a stable PTH of 372 pg/mL, whereas in patient 2 PTH spiked to over 5,000 pg/mL before falling to 274 pg/mL by day 90 (13). These results suggest that, beyond being elevated, the PTH kinetics after administration of denosumab cannot be easily predicted, further complicating interpretation of intraoperative PTH.
Our experience underscores the potential for denosumab to obscure or mimic parathyroid pathology. Our patient’s PTH increased from 209 to 465 pg/mL 14 days after administration of denosumab. After removal of one gland, PTH was 200 pg/mL at 10 minutes, meeting the Miami criterion (20). Supraphysiologic plateau at this range prompted concern for multiglandular disease, denosumab-induced alterations, or both. Exploration of remaining glands and subsequent 3.5 gland removal did decrease the PTH values into the normal range, however we urge surgeons to exercise caution in the management of patients who have received denosumab, as this introduces a confounder in the interpretation of intraoperative PTH. Furthermore, for patients with PHPT considering surgical intervention, denosumab administration should likely be delayed.
Conclusions
We have described a difficult case of parathyroidectomy in a postmenopausal woman who received denosumab for osteoporosis with a subsequent spike in PTH and altered intraoperative PTH kinetics. Further characterization of the effects of denosumab on calcium and PTH is warranted, particularly for patients who are considered for surgical management of parathyroid disease. The patient reported that the surgery and recovery went very well and was thankful for the care provided.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/aot-20-64
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-64). JOR, MD is a consultant for Baxter Scientific. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilezikian JP. Primary hyperparathyroidism. J Clin Endocrinol Metab 2018;103:3993-4004. [Crossref] [PubMed]
- Bilezikian JP, Bandeira L, Khan A, et al. Hyperparathyroidism. Lancet 2018;391:168-78. [Crossref] [PubMed]
- Stein EM, Silva BC, Boutroy S, et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J Bone Miner Res 2013;28:1029-40. [Crossref] [PubMed]
- De Geronimo S, Romagnoli E, Diacinti D, et al. The risk of fractures in postmenopausal women with primary hyperparathyroidism. Eur J Endocrinol 2006;155:415-20. [Crossref] [PubMed]
- Khosla S, Melton LJ, Wermers RA, et al. Primary hyperparathyroidism and the risk of fracture: a population‐based study. J Bone Miner Res 1999;14:1700-7. [Crossref] [PubMed]
- Amgen Inc. Prolia (denosumab) [package insert]. U.S. Food and Drug Administration website. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125320s186lbl.pdf. Revised May 2017. Accessed August 20, 2020.
- Cummings SR, Martin JS, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756-65. [Crossref] [PubMed]
- Diab DL, Watts NB. Denosumab in osteoporosis. Expert Opin Drug Saf 2014;13:247-53. [Crossref] [PubMed]
- Pittman K, Antill YC, Goldrick A, et al. Denosumab: Prevention and management of hypocalcemia, osteonecrosis of the jaw and atypical fractures. Asia Pac J Clin Oncol 2017;13:266-76. [Crossref] [PubMed]
- Strickling J, Wilkowski MJ. Severe, Symptomatic Hypocalcemia due to Denosumab Administration: Treatment and Clinical Course. Case Rep Nephrol Dial 2019;9:33-41. [Crossref] [PubMed]
- Nakamura Y, Suzuki T, Kamimura M, et al. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res 2017;5:17021. [Crossref] [PubMed]
- Makras P, Polyzos SA, Papatheodorou A, et al. Parathyroid hormone changes following denosumab treatment in postmenopausal osteoporosis. Clin Endocrinol (Oxf) 2013;79:499-503. [Crossref] [PubMed]
- Jang SM, Anam S, Pringle T, et al. Contrasting PTH Response of Denosumab Use in Dialysis Patients: A Report of 2 Cases. Pharmacy (Basel) 2020;8:59. [Crossref] [PubMed]
- Torregrosa JV. Dramatic increase in parathyroid hormone and hypocalcaemia after denosumab in a kidney transplanted patient. Clin Kidney J 2013;6:122. [Crossref] [PubMed]
- Bhanot RD, Kaur J, Bhat Z. Severe hypocalcemia and dramatic increase in parathyroid hormone after denosumab in a dialysis patient: a case report and review of the literature. Case Rep Nephrol 2019;2019:3027419 [Crossref] [PubMed]
- Sirvent AE, Enríquez R, Sánchez M, et al. Extreme hypocalcaemia and hyperparathyroidism following denosumab. Is this drug safe in chronic kidney disease? Nefrologia 2014;34:542-4. [PubMed]
- Salim SA, Nair LR, Thomas L, et al. Denosumab-associated severe hypocalcemia in a patient with chronic kidney disease. Am J Med Sci 2018;355:506-9. [Crossref] [PubMed]
- Hanley DA, Adachi JD, Bell A, et al. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 2012;66:1139-46. [Crossref] [PubMed]
- Block GA, Bone HG, Fang L, et al. A single‐dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012;27:1471-9. [Crossref] [PubMed]
- Irvin GL, Dembrow VD, Prudhomme DL. Clinical usefulness of an intraoperative "quick parathyroid hormone" assay. Surgery 1993;114:1019-22; discussion 1022-3. [PubMed]
Cite this article as: Tanavde V, Hondorp B, Russell JO. Parathyroidectomy in a patient treated with denosumab: a case report. Ann Thyroid 2021;6:12.


