Radiofrequency for benign thyroid nodules—critical review of the literature
Introduction
Radiofrequency ablation (RFA) has been used for tumors of several organs for a long time. Recently there has been more published data on its use for thyroid nodules (1-4). The purpose of this review article is to compile and comprehensively discuss the basics of the technique, review literature on its current use, and discuss future prospects of its use for benign thyroid nodule (BTN). Due to novelty of RFA for BTN, the use of this technique warrants expertise before it may be effectively performed by a physician. In addition, this new technique opens a broad field of research for the use of a non-surgical approach to benign, undetermined, and malignant thyroid nodules.
Radiofrequency
The radiofrequency generator produces a high frequency alternate current ranging from 200 to 1,200 KHz. This creates an oscillating electric field that increases the movement of ions and therefore generates heat (1). This frictional mechanism of heating subsequently increases the tissue temperature which is the final goal of RFA.
Radiofrequency is delivered to the desired tissue via an electrode which usually measures between 16-18G and is internally cooled. The major technical variable is the active tip’s size. This area of the electrode is the uncoated metal portion that interacts with the tissue. The active tip size has to be adequate in comparison to the size of the targeted lesion as it affects the amount of energy delivered. The power required for the tip to be effective depends on its size. For example, 5 mm tip operates with 15–20 W, 10 mm requires 30–50 W, whereas 15 mm needs 50–70 W (5).
The aim of thermal ablation is to kill tumor cells through heat. Temperatures below 40 °C do not disrupt homeostasis, however when the temperature reaches between 42–45 °C (hyperthermia), cells are more susceptible to damage by chemotherapy or radiotherapy. When temperature increases up to 46 °C for a duration of 60 min, irreversible cellular damage occurs. Further increasing the temperature up to 50–52 °C for 4–6 min causes cytotoxicity. However, instantaneous protein coagulation occurs once the temperature is between 60–100 °C causing destruction of cytosolic, mitochondrial, and nuclei proteins. This process will eventually lead to coagulative necrosis within several days. Scarring of the tissues starts once temperature reaches above 105 °C, unfortunately this will result in decreased energy conduction within the tissue (1,6,7).
Transmission of heat to the target lesion is key for effective ablation. Therefore, since the introduction of RFA there have been several modifications to the initial devices. A phenomenon known as heat-sink hampers the effectiveness of ablation. This occurs when the target lesion is in close proximity to a blood vessel which has a cooling effect and dissipates heat. As a result, the energy delivered is not concentrated at a certain point. However, this phenomenon can be utilized advantageously by using internally cooled electrodes, where the heat energy is at the periphery and is dispersed further, and thus increasing the ablation area. Overall, heat-sink plays a decisive role in the ablation process (1,6,7) (Figure 1).
Indications for RFA
BTNs are being diagnosed at an increasing rate due to wide use of ultrasound imaging. Majority of these nodules are small and, in approximately 85% of the cases, do not increase in size (8). However, some nodules do grow, and this depends largely on their initial size at the time of diagnosis (8,9). Some of these nodules become symptomatic and lead to cosmetic problems, warranting treatment with RFA.
Symptoms are accessed using a visual analog scale measuring 10 cm with a score ranging from 0–10. In comparison, the cosmetic scale is measured by the physician (1 - no palpable mass, 2 - palpable mass, no cosmetic problem, 3 - cosmetic problem while swallowing, 4 - readily detected cosmetic). No cutoff size to perform RFA is currently defined (4). However, RFA is used for nodules exceeding 2 cm in size or continuously growing at follow up or resulting in compressive symptoms with either cosmetic or clinical concern (4,10) (Table 1).

Full table
The benign nature of the nodule has to be determined by fine needle aspiration (FNAC) or core needle biopsy (CNB). The initial recommendations are that two samples needs to be done before RFA (two FNAC, or one FNAC followed by CNB) (11-13). Though, one pathologic sample can be acceptable when the nodule has ultrasonographic features highly specific for being benign (isoechoic spongiform nodule or partially cystic with intra cystic comet tail artifact) or it is an autonomous functioning thyroid nodule (AFTN) (4,10,12,13).
Ultrasound assessment
Patients with thyroid nodules should have a thyroid and cervical ultrasound exam in order to access the risk of malignancy. The ultrasound is done to answer questions regarding the thyroid such as: Is there a distinct thyroid nodule? Does the nodule have benign characteristics? What is the nodule’s volume? What is the cystic percentage of the nodule? Are there suspicious lymph nodes? And the position of the nodules related to other structures (14).
It is preferred that the physician performing the procedure should do the patient’s assessment. This will be beneficial in anticipating technical difficulties during the procedure. It will also help to determine the number of ablation sessions required, selection of the tip size, and to assess the proximity of other structures at the target lesion. It is also advised to have a thorough discussion with the patient regarding the possible outcomes and risk of the procedure (4,14).
The color doppler, though not useful for malignancy stratification, can be useful for vascular ablation and ablation completeness assessment. Vascular ablation is a variation of the ablation technique that addresses a specific nutrient vessel which will reduce the risk of intranodal bleeding or minimize the heat-sink effect of highly vascularized nodules. Another use of the vascular assessment is inspection of vascularization after the ablation, since residual vascularization is one of the critical factors related to regrowth of nodules (15,16).
Pre-op workup
Additional exams such as complete blood count and coagulation tests are advisable. Thyroid function tests (TSH, free T4l) will assure that the procedure will not alter the remaining thyroid tissue function. Further testing depends on the patient, for example axial exams (CT or MRI) are required for sub sternal goiters. Evaluation of complete volume of the thyroid, especially of autonomous nodules, may be hindered when using only the ultrasound. Therefore, additional imaging techniques such as technetium 99mTc pertechnetate or a 123I thyroid may be useful for complete assessment.
Assessment of the cytological nature of the nodules is also important prior to RFA. Traditionally it is advised to perform two cytological Bethesda II FNACs or one cytology and one CNB. The need for both studies may be avoided when a benign exam is accompanied with ultrasound features suggestive of benign nodules (4). Currently, the use of molecular tests or gene expression classifier (GEC) is not broadly accepted as an indication for ablation of benign lesions (4,17-19).
Results on benign nodules
BTNs respond consistently to RFA. Typically, cystic nodules experience an increased volume reduction when compared to solid nodules (71% vs. 51% volume reduction ratio (VRR) during the first month P=0.000), although this difference is not statistically significant after 6 months (20). This can be partially explained by the aspiration of the cystic portion of the nodule during the procedure compared to the solid nodules. A recent systematic review demonstrated the role of thermal ablation in volume reduction and symptom relief. VRR of 71% was achieved at 12 months follow up (P<0.05) after one session of RFA (21). It was also reported that subsequent RFA sessions increased the VRR up to 87% at 24 months follow up (21).
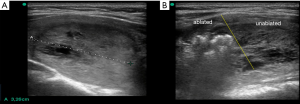
There are a few variables which affect the final VRR after patients undergo RFA. These variables include the initial nodule volume, ultrasonographic features of the nodule, and the number of RFA sessions. These factors influence the amount of viable tissue left behind. Post-RFA, the average VRR is reported to be between 64.9–93.5% (3,22-30). However, these variables may affect the final outcome (Figure 2).
Nodular size has been reported to affect the VRR post-RFA. Nodules greater than 30 ml show significantly lower VRR than the smaller ones (57% vs. 69%) at 6 months and 12 months (63% vs. 75% respectively) follow up (21,31,32). Rate of regrowth at an average follow up of 39 months is reported to be 24.1% (33). Regrowth is directly related to the effectiveness of the first ablation session and the initial size of the nodule.
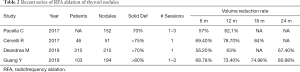
Some parameters have been reported to assess the efficacy of RFA. Sim et al. (34) defined the initial ablation rate (IAR) which is the volume of ablated tissue divided by the total volume of the nodule. IAR rates greater than 70% are expected to have VRR greater than 50% upon follow up (15). Vascular ablation can be used, as described by Park et al. (29,31-34) to further increase the ablation area (Table 2).
Full table
IAR may be increased by reducing the margin of unablated tissue around the nodule. This can be achieved through hydro dissection techniques once the physician becomes comfortable with the procedure and obtains thorough understanding of the difficulties and complications. It will also protect critical structures that might be injured by RFA (35) (Figure 3).
AFTN
The standard treatment of AFTN is surgery or radioiodine ablation (RAI). Both treatments entail drawbacks such as the inherent risks of surgery and hypothyroidism that can manifest after RAI. Since the first report of RFA for an AFTN (23) several prospective series have demonstrated its usefulness. Sung et al. reported significant VRR, increase in TSH, and complete normalization of thyroid function in 81% of the patients (5). Post-RFA thyroid function normalization is reported between 24–82% (36) and it correlates to nodule’s size. Cesareo et al. reported that nodules measuring below 10-13cc resulted in better normalization of thyroid function (37). Recently, a meta-analysis endorsed this inverse relationship between nodule size and normalization of thyroid, reporting the pooled rate as 57% (38).
The data referenced above supports the treatment of AFTN as neoplastic lesions, as opposed to with an intent of simply volume reduction. Therefore, all viable nodular tissue should be ablated as thoroughly as possible. Relatively, larger nodules are more challenging as some areas remain inaccessible during the first session of RFA. These technical challenges make RFA less effective when treating AFTN compared to RAI or surgery.
Cost-effectiveness
In addition to the proof of efficaciousness demonstrated above, a key question to be addressed is RFA’s cost-effectiveness. Yue et al. conducted a retrospective cohort analysis and reported a direct higher cost of RFA when compared to open thyroidectomy for benign nodules (US$ 2,740.00 vs. US$ 1,872.00 respectively) in China. However, all the domains of quality of life were better with RFA (39). Additionally, Deandrea et al. demonstrated that nodules below 15 ml developed higher volume reduction rates than nodules with volumes between 15-30ml or higher. Thus, the question is whether RFA should be initiated early in the nodule’s natural history (16). To conclude, in order to increase cost-effectiveness of RFA, one can either expect natural decrease of technology costs or select nodules for treatment which increase chances of better outcomes.
Thermal ablation technologies
There are two main available thermal ablation technologies: RFA and Light Amplification by Stimulated Emission of Radiation (LASER). Although there is little data comparing both technologies, a recent meta-analysis by Trimboli et al. (21) demonstrated a higher VRR with RFA in comparison to LASER at 6-, 12- and 24-months follow-up. However, some caution is needed while interpreting this data. First, the timeframe is different as studies with LASER are older with longer follow-up compared to RFA. Second, the initial nodule volume in RFA’s studies is smaller than the nodules which underwent LASER. Third, the LASER studies were mainly published on European population compared to RFA, which came out from Europe and South Korea. Therefore, further studies are needed comparing these two techniques in different environments and addressing the costs involved.
Discussion
RFA is a comparatively a safe and widely available technique. It has already been used to treat malignancies of other organs. In addition, extensive literature is available on biological interaction and physical properties which affect the procedure’s effectiveness such as heat-sink phenomenon with the use of RF. Thus, it can comfortably be used and will play a significant role in the management of thyroid nodules.
The indication of RFA for BTNs has been established by the Korean Radiology Society (4,11). These have been endorsed by other international groups (12,40), and used as inclusion criteria for almost every study on the subject. The cornerstone of the indication is the nodule pathology as after RFA the US features and cytological assessments are jeopardized. Currently, this is accomplished by two FNACs or one FNAC and one CNB (11). However, these criteria are becoming broader after the publication of second Korean guidelines (4,10). Uncertainty of Bethesda III and IV limits the use of RFA on thyroid nodules. Therefore, there is an expectation to move towards molecular tests or gene classifier tests, and including these in the criteria once they become readily available.
The results of RFA on benign thyroid nodules (BTNs) are well-defined in the literature (10,31,34,41,42). Significant reduction in nodule’s volume as well as cosmetic and symptomatic scores is reported. In addition, thyroid function preservation which is a desirable outcome of RFA is also mentioned. Therefore, this minimally invasive procedure is rapidly gaining acceptance outside South Korea due to increased benefits along with lower complications rates when compared to surgery (43,44).
Finally, RFA for thyroid is a specialized procedure that requires an expert physician as it involves critical steps and close follow up of patients. To this date, there are only a handful of medical centers that are teaching their residents and fellows on the use of RFA. This is largely because detailed knowledge of ultrasound handling, RFA technique, thyroid pathology, and neck anatomy are not fully mastered by the mentors (Figure 4). Thus, in order to perform RFA safely and effectively, every physician will have to seek avenues for building competency in the relevant fields.
Conclusions
RFA for thyroid nodule is a promising and new modality of treatment. Currently, an extensive literature is already available including guidelines to ensure the reproducibility and safety of the procedure. However, successful implementation of RFA programs for thyroid requires thorough understanding of all aspects of the technique including the indication, cost, complications and outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for the series “The Management of Thyroid Tumors in 2021 and Beyond”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-45). The series “The Management of Thyroid Tumors in 2021 and Beyond” was commissioned by the editorial office without any funding or sponsorship. Dr. JR served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 2001;13:129-47. [Crossref] [PubMed]
- Kim YS, Rhim H, Tae K, et al. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 2006;16:361-7. [Crossref] [PubMed]
- Deandrea M, Limone P, Basso E, et al. US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 2008;34:784-91. [Crossref] [PubMed]
- Kim JH, Baek JH, Lim HK, et al. 2017 Thyroid Radiofrequency Ablation Guideline: Korean Society of Thyroid Radiology. Korean J Radiol 2018;19:632-55. [Crossref] [PubMed]
- Sung JY, Baek JH, Jung SL, et al. Radiofrequency ablation for autonomously functioning thyroid nodules: a multicenter study. Thyroid 2015;25:112-7. [Crossref] [PubMed]
- Rhim H, Goldberg SN, Dodd GD 3rd, et al. Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics 2001;21:S17-35; discussion S6-9. [Crossref] [PubMed]
- Shin JH, Baek JH, Ha EJ, et al. Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012;2012:919650 [Crossref] [PubMed]
- Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA 2015;313:926-35. [Crossref] [PubMed]
- Seo SH, Kim TH, Kim SH, et al. Predicting the Size of Benign Thyroid Nodules and Analysis of Associated Factors That Affect Nodule Size. Chonnam Med J 2015;51:97-101. [Crossref] [PubMed]
- Kim JH, Baek JH, Lim HK, et al. Summary of the 2017 thyroid radiofrequency ablation guideline and comparison with the 2012 guideline. Ultrasonography 2019;38:125-34. [Crossref] [PubMed]
- Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 2012;13:117-25. [Crossref] [PubMed]
- Negro R, Attanasio R, Grimaldi F, et al. A 2016 Italian Survey about Guidelines and Clinical Management of Thyroid Nodules. Eur Thyroid J 2017;6:75-81. [Crossref] [PubMed]
- Garberoglio R, Aliberti C, Appetecchia M, et al. Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound 2015;18:423-30. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Sim JS, Baek JH, Cho W. Initial Ablation Ratio: Quantitative Value Predicting the Therapeutic Success of Thyroid Radiofrequency Ablation. Thyroid 2018;28:1443-9. [Crossref] [PubMed]
- Deandrea M, Garino F, Alberto M, et al. Radiofrequency ablation for benign thyroid nodules according to different ultrasound features: an Italian multicentre prospective study. Eur J Endocrinol 2019;180:79-87. [Crossref] [PubMed]
- Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 2013;269:293-300. [Crossref] [PubMed]
- Kim C, Lee JH, Choi YJ, et al. Complications encountered in ultrasonography-guided radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers. Eur Radiol 2017;27:3128-37. [Crossref] [PubMed]
- Jung SL, Baek JH, Lee JH, et al. Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study. Korean J Radiol 2018;19:167-74. [Crossref] [PubMed]
- Jeong WK, Baek JH, Rhim H, et al. Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 2008;18:1244-50. [Crossref] [PubMed]
- Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine 2020;67:35-43. [Crossref] [PubMed]
- Aysan E, Idiz UO, Akbulut H, et al. Single-session radiofrequency ablation on benign thyroid nodules: a prospective single center study: Radiofrequency ablation on thyroid. Langenbecks Arch Surg 2016;401:357-63. [Crossref] [PubMed]
- Baek JH, Jeong HJ, Kim YS, et al. Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid 2008;18:675-6. [Crossref] [PubMed]
- Baek JH, Moon WJ, Kim YS, et al. Radiofrequency ablation for the treatment of autonomously functioning thyroid nodules. World J Surg 2009;33:1971-7. [Crossref] [PubMed]
- Baek JH, Kim YS, Lee D, et al. Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 2010;194:1137-42. [Crossref] [PubMed]
- Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 2011;12:525-40. [Crossref] [PubMed]
- Spiezia S, Garberoglio R, Milone F, et al. Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 2009;19:219-25. [Crossref] [PubMed]
- Lee JH, Kim YS, Lee D, et al. Radiofrequency ablation (RFA) of benign thyroid nodules in patients with incompletely resolved clinical problems after ethanol ablation (EA). World J Surg 2010;34:1488-93. [Crossref] [PubMed]
- Huh JY, Baek JH, Choi H, et al. Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session--prospective randomized study. Radiology 2012;263:909-16. [Crossref] [PubMed]
- Cesareo R, Pasqualini V, Simeoni C, et al. Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab 2015;100:460-6. [Crossref] [PubMed]
- Deandrea M, Trimboli P, Garino F, et al. Long-Term Efficacy of a Single Session of RFA for Benign Thyroid Nodules: A Longitudinal 5-Year Observational Study. J Clin Endocrinol Metab 2019;104:3751-6. [Crossref] [PubMed]
- Cesareo R, Palermo A, Pasqualini V, et al. Efficacy and safety of a single radiofrequency ablation of solid benign non-functioning thyroid nodules. Arch Endocrinol Metab 2017;61:173-9. [Crossref] [PubMed]
- Sim JS, Baek JH, Lee J, et al. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia 2017;33:905-10. [Crossref] [PubMed]
- Sim JS, Baek JH. Long-Term Outcomes Following Thermal Ablation of Benign Thyroid Nodules as an Alternative to Surgery Endocrinol Metab (Seoul) 2019;34:117-23. [Crossref] [PubMed]
- Rangel LG, Volpi EM, Steck JH, et al. Radiofrequency Ablation Systemization. VideoEndocrinology 2020. doi:
10.1089/ve.2020.0175 . - Bernardi S, Stacul F, Michelli A, et al. 12-month efficacy of a single radiofrequency ablation on autonomously functioning thyroid nodules. Endocrine 2017;57:402-8. [Crossref] [PubMed]
- Cesareo R, Naciu AM, Iozzino M, et al. Nodule size as predictive factor of efficacy of radiofrequency ablation in treating autonomously functioning thyroid nodules. Int J Hyperthermia 2018;34:617-23. [Crossref] [PubMed]
- Cesareo R, Palermo A, Benvenuto D, et al. Efficacy of radiofrequency ablation in autonomous functioning thyroid nodules. A systematic review and meta-analysis. Rev Endocr Metab Disord 2019;20:37-44. Erratum in: Rev Endocr Metab Disord 2 2019;20:45. [Crossref] [PubMed]
- Yue WW, Wang SR, Li XL, et al. Quality of Life and Cost-Effectiveness of Radiofrequency Ablation versus Open Surgery for Benign Thyroid Nodules: a retrospective cohort study. Sci Rep 2016;6:37838. [Crossref] [PubMed]
- Dobnig H, Zechmann W, Hermann M, et al. Radiofrequency ablation of thyroid nodules: "Good Clinical Practice Recommendations" for Austria: An interdisciplinary statement from the following professional associations: Austrian Thyroid Association (ÖSDG), Austrian Society for Nuclear Medicine and Molecular Imaging (OGNMB), Austrian Society for Endocrinology and Metabolism (ÖGES), Surgical Endocrinology Working Group (ACE) of the Austrian Surgical Society (OEGCH). Wien Med Wochenschr 2020;170:6-14. [Crossref] [PubMed]
- Cesareo R, Palermo A, Pasqualini V, et al. Radiofrequency ablation for the management of thyroid nodules: A critical appraisal of the literature. Clin Endocrinol (Oxf) 2017;87:639-48. [Crossref] [PubMed]
- Hamidi O, Callstrom MR, Lee RA, et al. Outcomes of Radiofrequency Ablation Therapy for Large Benign Thyroid Nodules: A Mayo Clinic Case Series. Mayo Clin Proc 2018;93:1018-25. [Crossref] [PubMed]
- Hong MJ, Sung JY, Baek JH, et al. Safety and Efficacy of Radiofrequency Ablation for Nonfunctioning Benign Thyroid Nodules in Children and Adolescents in 14 Patients over a 10-Year Period. J Vasc Interv Radiol 2019;30:900-6. [Crossref] [PubMed]
- Choi Y, Jung SL, Bae JS, et al. Comparison of efficacy and complications between radiofrequency ablation and repeat surgery in the treatment of locally recurrent thyroid cancers: a single-center propensity score matching study. Int J Hyperthermia 2019;36:359-67. [Crossref] [PubMed]
Cite this article as: Rangel LG, Volpi E, Steck JH, Volpi LM, Russell J, Muhammad H, Tufano RP. Radiofrequency for benign thyroid nodules—critical review of the literature. Ann Thyroid 2021;6:2.