
Impact of healthcare resources on management of indeterminate thyroid tumors
Introduction
Thyroid nodules are described as distinct formations within the thyroid that are radiologically separated from the surrounding thyroid tissue. These nodules are being encountered more and more in clinical practice, occurring in at least 33% of the population (1). The priority in the management the thyroid nodules is to differentiate between the symptomatic (compressive symptoms like dyspnea, dysphonia or dysphagia, or thyroid dysfunction) and suspicious for malignancy (SFM) (accounting for nearly 10%) cases and the others. Studies have shown that nearly 95% of thyroid nodules are benign, and generally do not transform into a malignant disease (2,3). They are usually detected in asymptomatic patients who are being examined for other medical reasons. The incidental detection of thyroid nodules frequently precedes a diagnosis of malignancy. The biopsy of all detected nodules is unreasonable, while an overly conservative attitude toward biopsy can result in a missed diagnosis of a thyroid cancer.
Proper management is vital for the prevention of unnecessary thyroidectomies for asymptomatic nodules with no malignancy, and delayed or missed diagnoses, and for the management of thyroid malignancy. Updated international guidelines, such as those of the American Association of Clinical Endocrinologists, the American Thyroid Association (ATA), Associazione Medici Endocrinology, the British Thyroid Association, the American College of Endocrinology and the National Comprehensive Cancer Network, offer similar suggestions for the management of nodules in the thyroid (Figure 1). The common approaches have been systematically repeated by these professional associations from around the world, and remained all but unchanged for some considerable time. Management, however, comes with a significant disadvantage, in that nearly 25% of all FNABs for thyroid nodules do not give cytological results pointing to a firm diagnosis.
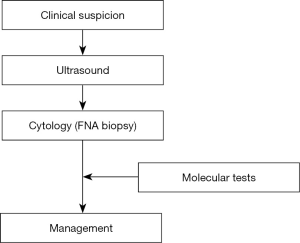
Data from recent studies show that the attentive selection of nodules to aid biopsy should be based on an evaluation of risk of malignancy (ROM). The high-risk characteristics of thyroid malignancy and nodule size detectable on ultrasonography (USG) can help assess the need for fine needle aspiration biopsy (FNAB). FNAB is an important tool for assessment of thyroid nodules, the results of which must be communicated in an agreed and clear language between the pathologist and the clinician.
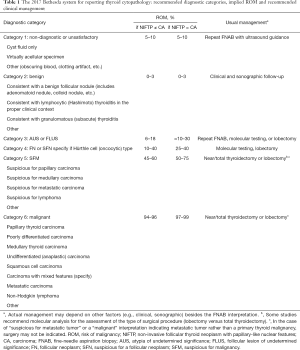
As stated above, most nodules are benign, and are managed by clinical observation, while malignant nodules usually require surgical resection. In this regard, a good differential diagnosis between malignant and benign nodules is of vital importance in clinical management. FNAB is a dependable and cost-effective tool for the clarification of the character of the thyroid nodule. While FNAB is used today as a matter of routine, cytopathologists have encountered problems in communicating and reporting the results to their colleagues. Since October 2007, with the introduction of the Bethesda Thyroid Cytopathology Reporting System (TBSRTC), some significant reporting problems related to thyroid FNA samples and the problems of communicating the results between clinicians and cytopathologists have been resolved. TBSRTC is largely accepted by many institutions throughout the world. The 2017 revision of TBSRTC was inspired by new data and improvements in the area of thyroid pathology, such as revised international guidelines for thyroid nodules, the use of molecular tests ancillary to cytopathologic investigations, and the recategorization of the non-invasive “follicular variant of papillary thyroid carcinoma” as “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” (4,5). TBSRTC categorized the reporting of thyroid FNAB into six categories, and has recently been very successful in predicting ROM for each category. Table 1 shows the six original general categories that have been retained in the 2017 revision of the TBSRTC (6).
Full table
In this review, we present the impact of healthcare resources on the management of indeterminate thyroid tumors (ITTs) and the cost-effectiveness of the diagnosis and management options of them. Data regarding “the indeterminate nodule” have also been reviewed to make this complicated topic of the study easier to understand.
Diagnosis and evaluation of ITTs
Despite the introduction of the TBSRTC, FNAB can still lead to a substantial number of uncertain results due to the common cytologic features between benign and a particular subcategory of malignant nodules. The TBSRTC includes three indeterminate categories with different ROM, being atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) (TBSRTC category III); follicular neoplasm/suspicious for a follicular neoplasm (FN/SFN) or Hürthle cell neoplasm-suspicious for Hürthle cell neoplasm (TBSRTC category IV); and SFM (TBSRTC category V).
Usually, TBSRTC category III includes samples containing follicular cells with nuclear and/or structural atypia, which are inadequate to be diagnosed as SFM or FN. AUS/FLUS has been examined extensively, but predicting the ROM has been challenging. The TBSRTC recommends limiting usage of the AUS/FLUS terms to around 10% or lower of all FNABs. AUS/FLUS has been named as the “gray zone” in FNAB cytology. The purpose of the AUS/FLUS category was to decrease unnecessary surgeries, and for this reason, attempts are made to define the ROM in this category. The ROM of AUS/FLUS has been shown to be not high, but varies considerably from study to study, ranging from 5% to 48% (7), and the different cytologic subtypes of AUS/FLUS are assumed to be the main cause of this variable range of ROM.
The terms FN and SFN are synonymous, and so should not be used to report two different results. The FN category includes follicular cancers, follicular adenomas, follicular variant of papillary thyroid carcinomas and some hyperplastic adenomatous nodules.
The TBSRTC has brought molecular testing into use as an ancillary diagnostic tool for the FNAB cytology, as a means of avoiding unnecessary operations (6). Both the European Thyroid Association and ATA immediately accepted this suggestion, and so emphasize the importance of these tests on the FNAB of the thyroid (5,8,9). Different molecular tests have been improved and have already earned a place in the suggested assessment of indeterminate tumors in the National Comprehensive Cancer Network and ATA guidelines, which were revised in 2015 (1,9). That said, other international guidelines, such as the British Thyroid Association, Associazione Medici Endocrinology, American College of Endocrinology and American Association of Clinical Endocrinologists, have been more prudent in suggesting the usage of molecular tests for the management of ITTs (8,10). The reason for this more conventional attitude is the deficiency of wide prospective studies; the significant cost of these tests (most largely used tests are >US $5,000 per nodule); and their unrecognized long-term effect on healthcare resources and cost-effectiveness (10). Currently, there are variously molecular tests available with different negative predictive values and positive predictive values, although there is still a lack of consensus on the optimum molecular strategy. The thyroid tumor prevalence and the cancer rates in each TBSRTC category in each institution can affect molecular test outcomes. Furthermore, the majority of molecular tests are trademarked and concentrated in the United States, and the European healthcare systems don’t refund them.
Molecular tests have shown that evaluations of ROM of nodules must be individualized. Investigating the ROM of every indeterminate category in each healthcare center must be the first step in the proper evaluation of molecular tests, as numerous other causes can affect the individual pretest ROM of a nodule. As FNAB may give indeterminate results, molecular tests can be utilized for the differential diagnosis of patients with an indeterminate FNAB cytology that require resection and those who may be managed more conventionally. However, molecular tests must not only be correct and dependable, but also maintainable for the best results.
Despite the usage of thyroid USG and FNAB continues to rise rapidly, the number of ITTs will follow those curves.
Factors for ROM stratification of ITTs
Clinical features
Some risk factors, such as palpable cervical lymph nodes; stiffness of the nodule on physical examination with fixation to adjacent tissues; rapid and painful expansion; presence of compressive symptoms like dyspnea, dysphonia or dysphagia; or recurrent laryngeal nerve dysfunction, although infrequent, increase the ROM considerably, and may lead the clinician to suggest a diagnostic thyroidectomy, in spite of an indeterminate FNAB result (11). Exposure to radiation; presence of thyroid cancer in the family; male sex; solitary nodules; and nodule size >4 cm are the other risk factors that can moderately raise the ROM of nodules (12).
Ultrasonographic features and other imaging modalities
Thyroid USG has been largely utilized for the discovery of non-palpable nodules, for predicting the ROM of nodules, and for defining the features of cervical lymph nodes and nodules. Studies have shown that USG findings SFM include hypoechogenicity, microcalcifications, an egg shape, and irregular or unclear margins. That said, whether the usage of these USG features is helpful in ITTs continues to be unclear. A meta-analysis revealed that only increased intranodular vascularity predicted thyroid cancer, while other suspicious USG features were not useful in this regard in ITTs (13). ITTs with a “low suspicion” or “intermediate suspicion” appearance on USG according to the ATA had cancer prevalence of between 14% and 31% with matching outcomes in these categories. International guidelines confirm that the ultrasonographic view should effect the management of the thyroid nodules to some degree, but as yet offer no other management approach to ITTs with miscellaneous ultrasonographic characters.
In a systematic review investigating the efficacy of fluorodeoxyglucose-positron emission tomography fluorodeoxyglucose-positron emission tomography for the diagnosis of thyroid malignancy, the specificity was 95% and sensitivity was 48% (14). Accordingly, an ITT that uptakes fluorodeoxyglucose with a high standardized uptake value (SUV) is not always thyroid cancer, but thyroid cancers seldom don’t uptake fluorodeoxyglucose. Fluorodeoxyglucose-positron emission tomography scans have wide feasibility, but the problems of low-resolution counts for small tumors, unreliable descriptions of fluorodeoxyglucose avidity, high cost and selection bias are notable disadvantages.
The elastography method on USG uses compression on the tissue, from which it calculates the level of stretching of the tissue to estimate its hardness. This imaging technique is used to differentiate between malignant and benign nodules by evaluating their elasticity. Rago et al. reported that the maximum elasticity scores were always related with cancer, with 97% sensitivity and 100% specificity (15). USG elastography is a new and developing method for the management of the indeterminate nodules, although wider prospective studies are required to determine the diagnostic accuracy of this procedure.
Cytological features
The evaluation of an FNAB is still the most precise single investigation for the management of nodules in the thyroid. Table 2 shows the TBSRTC categories and the categories of other classification systems in the same frame. TBSRTC category VI or TBSRTC category II have a ROM of around >97% and <5%, respectively, and these rates make these two categories quite dependable, although around 25% of FNAB cytologies result in an indeterminate cytology (19,20). Each TBSRTC category is associated with an estimated ROM, as shown in Table 1, but these ROMs are highly varied in the publications of different centers, ranging from 42% to 90% in TBSRTC category V, 14% to 49% in TBSRTC category IV and 6% to 48% in TBSRTC category III (21,22).

Full table
The recategorization of the non-invasive follicular thyroid neoplasm with papillary-like nuclear features both leads to an admission of borderline tumors and changes the terminology of a number of thyroid tumors. TBSRTC categories III and IV can include these borderline lesions rather than those indicating benign or malignant tumors.
The usage of both ultrasonographic appearance and cytological subcategories together improves the ROM stratification of ITTs, and supports the estimation of a histological result.
The cost of FNAB can affect FNAB repeat decisions, especially in AUS/FLUS cases. The cost of FNAB varies widely between different countries. For instance, while it ranges between $120–$140 in Italy, it is $101.4 in Turkey, $11 in Nigeria, $1,017 in New Zealand (radiologist-performed), 23.73 R$ (about $7) in Brasil and about $20 in Japan (23-27). In countries where the cost of FNAB is low, repeating an FNAB before other diagnostic methods for AUS/FLUS can be considered a reasonable choice, as it can prevent unnecessary surgeries. In contrast, it is not surprising that in countries where the cost of FNAB is high, diagnostic surgery may be preferred over a repeat FNAB.
The role of molecular genetics
In healthcare systems with publicly-funded health insurance plans that provide coverage to all members of the public, social responsibility is an important factor to be considered in the provision of healthcare services, and in particular, new diagnostic methods. As surgery poses a significant cost burden to the healthcare system (pre-, intra- and post-operative costs, lifelong medication, potential complications, etc.), any decrease in the number of diagnostic surgeries may translate to important cost savings in healthcare resources. Molecular testing is a less-invasive alternative diagnostic tool to diagnostic surgery. Although there are costs related with molecular tests, avoiding an unnecessary thyroidectomy may provide important cost savings in the long-term and may increase the quality of life of the patients by sparing them from lifelong medications and the potential complications associated with thyroidectomy.
The ThyGenX/ThyraMIRTM (Interpace Diagnostics, Parsippany, New Jersey), the Afirma Gene Sequencing ClassifierTM (Veracyte, Inc., South San Francisco, CA, USA) and the ThyroSeqTM (University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, and CBLPath, Inc., Rye Brook, NY, USA) are the three molecular tests that are currently available in the private sector for ITTs, and all are nucleic acid-based. Their working principles include genotyping for tumor-associated driver mutations and gene fusions, and expression profiling for a panel of genes, or a combination of both.
Molecular studies have gained acceptance in the United States as a popular means of identifying ITTs, and are seen as a potentially practice-changing method. Nearly 40% of all papillary thyroid carcinomas, 33% of some poorly differentiated cancers and 45% of anaplastic malignancies can carry a BRAF gene (V600E) mutation (28). Gene expression tests and mutational tests are the most frequently approaches, and only FNAB material is sufficient for both to study.
Mutational analysis
For a mutational analysis, firstly, genetic material is derived from the FNAB sample and DNA sequencing is carried out. Potential mutations in TP53, TERT, RAS, BRAF, and other related genes are sought, and fusion genes like RET/PTC and PAX8/PPARG are scanned. These are referred to as “rule in tests”, as if these tests are positive, a malignancy in the thyroid is found nearly every time, aside from in the presence of RAS mutations. RAS gene family (NRAS, KRAS, HRAS) mutations can be found in some papillary thyroid carcinomas (13%), follicular thyroid cancers (40–50%), benign follicular nodules (20–40%) and in non-invasive follicular thyroid neoplasm with papillary-like nuclear features (30%), and so are less specific (4,28). It should be noted that failure to detect a mutation in a mutational analysis does not rule out thyroid cancer. Cancer with mutations may have been missed in nearly 4% of cases in tests, so it should be considered that there may be false negative and positive outcomes, especially when RAS mutations are detected.
In a study conducted by Nikiforov et al., in AUS/FLUS and FN/SFN categories, mutational analysis (ThyroSeq v2) gave a 96% negative predictive value when no mutation was detected, and a positive predictive value of nearly 80% (29). In another single-center study, including 182 patients with a 190 TBSRT category 3 or 4 cytology, Valderrabano et al. reported a negative predictive value in the mutational test of 91%, while the positive predictive value was 42% (30).
Gene expression analysis/gene expression classifier (GEC)
This second type of molecular study investigates the presence of specific genes from among a panel of 142 genes, and differentiate between benign or SFM categories, but not malignancy. GEC detects nodules in the thyroid that can be managed without thyroidectomy, and so it is referred to as a “rule-out test”. In a study by Alexander et al., it was shown that the negative predictive value in an Afirma test was around 95% when it resulted in a benign finding (31). In a retrospective cohort study, the negative predictive value of GEC was found to be 92%, and the malignancy prevalence to be 31% (32).
Clinical management recommendations for ITTs
AUS/FLUS
AUS/FLUS should be diagnosed and managed by a multidisciplinary team comprising radiologists, endocrinologists, pathologists and surgeons. Repeat FNAB, molecular studies, core needle biopsy and active surveillance are the management alternatives that are recommended by international guidelines and in recently published studies. The AUS/FLUS category has incidence and cancer rates over a very wide range, so while managing this category, geographical and institutional rates should be considered. A repeat FNAB can result in a benign cytology diagnosis in up to 50% of nodules in which the initial FNAB evaluation was AUS/FLUS. Therefore, it should be chosen in nodules without suspicious clinical findings and ultrasonographic characteristics (33). There have been some studies reporting that a repeat FNAB had no effect on the thyroid cancer rate, whereas others have reported opposite results. The TBSRTC suggests FNAB in the AUS/FLUS category, with ATA makes no such absolute recommendation (making only a weak recommendation) in this category. In such cases, the ETA, Associazione Medici Endocrinology and American Association of Clinical Endocrinologists guidelines suggest that repeat FNABs may cause confusion (34,35). Molecular studies can absolutely take into account if required, and their accuracy is closely associated with the prevalence of thyroid malignancy in the population in which the test is performed (8). Among the molecular tests, when the GEC results were benign or the ThyroSeq results were negative, the ROM in this category was between 3% and 4%. The recently updated National Comprehensive Cancer Network guidelines state that active surveillance can be considered if the ROM of an ITT can be reduced to the level of a benign tumor (36). When the GEC molecular test is suspicious or a mutational analysis is positive, a diagnostic thyroidectomy should be performed in the AUS/FLUS category. Some researchers have questioned the cost-effectivity of molecular studies in comparison to repeat FNAB or other approaches to the management of the AUS and FLUS cytologies, while others have suggested a more conservative attitude in indeterminate FNAB cytologies with a benign genetic result.
Some argumentative studies have reported core needle biopsy to be more beneficial in the management of AUS/FLUS cases than repeat FNAB (37), although repeat FNAB together with molecular tests is suggested for the advanced investigation of AUS/FLUS nodules.
FN/SFN
This category of TBSRTC has a ROM after resection of 20–30% (20), and for this reason, a diagnostic thyroidectomy is suggested for routine management. The malignancy prevalence affects the ROM, although the ROM is around 6% when the GEC molecular test is benign, and between 3% and 4% when the ThyroSeq molecular test is negative in this category (38). When the molecular tests are negative, active surveillance can be selected from among the management options of FNs, although there are insufficient long-term follow-up studies to detect their behavior. When molecular tests are positive or the gene expression analysis is suspicious, diagnostic surgery should be considered (38). This category also contains Hürthle cell tumors, however there are a lack of specific studies to allow a generalization of the results of molecular studies. There have been a few studies to date recommending that USG-guided core needle biopsy can help reduce the frequency of insufficient diagnostic results, and increase the rate of accurate diagnoses. The USG-guided core needle biopsy approach is safe and is well-tolerated, gives low complication rates, and is an appropriate alternative to FNAB for the obtaining of tissues for diagnosis. Its outcomes, however, are indeterminate for up to 36% of thyroid nodules whose cytology is insufficient for the differentiation of nodular hyperplasia from FN (19).
While the ROM of FN is between 10% and 20% in Western countries, it is between 12.4% and 15.9% in Japan, meaning that FNA alone cannot adequately differentiate between nodules that require a diagnostic thyroidectomy. This is why other diagnostic tools are also selected to support decision-making in the management of indeterminate A (FN) nodules in Japan (39).
The FLUS category of the TBSRTC corresponds to the subcategory A-1, favor benign, in the Japan Thyroid Association system, and it has been suggested that diagnostic thyroidectomies should not be performed in this subcategory based on cytological results alone. Concerning the indeterminate B subcategory in the Japan Thyroid Association system, repeat FNAB or active surveillance is suggested, like in the AUS category of the TBSRTC (39).
SFM
This category has a ROM of 60–75%, which is the highest ROM ratio among the indeterminate categories in the TBSRTC (20). The ATA guidelines recommend that a diagnostic thyroidectomy should be carried out in the presence of SFM nodules due to the high ROM (5). Unfortunately, molecular tests cannot help in this category. The ROM in the “malignancy suspected” category is almost equal to that in the malignancy category in Japan, and so is classified in the same category as malignancy in the Japan Thyroid Association classification system, and a diagnostic thyroidectomy is performed when detected (39).
According to the 2015 ATA guidelines, active surveillance can be chosen from among the management options for tumors with very low risk, such as papillary microcarcinomas with no local invasion and distant metastases, and when there are no findings of aggressive cancer; in patients with a high level risk for surgery; in patients with an expected short life span due to serious disease, other cancers or very advanced age; or in patients with simultaneous surgical and/or medical issues that should be considered prior to surgery (5). The Korean Thyroid Association makes the same recommendation for selective papillary microcarcinomas as an alternative management option, especially for patients of advanced age, in line with ATA 2015 (40). Other guidelines, such as British Thyroid Association and American Association of Clinical Endocrinologists, are yet to include active surveillance in their guidelines. Consequently, nodules that have been biopsied and that are classified as “suspicious for papillary thyroid carcinoma” cytology, and that meet the active surveillance criteria of the guidelines for papillary thyroid carcinoma, can be considered for an active surveillance management approach.
Diagnostic surgery for ITTs
Total thyroidectomy and istmusectomy-added lobectomy are two surgical treatment options for ITTs. The advantages of lobectomy are the significantly lower risk of hypoparathyroidism, bilateral inferior laryngeal nerve injury and permanent hypothyroidism; while the disadvantage is that a completion thyroidectomy may be required if indicated. The advantage of the total thyroidectomy approach is preparation for radioactive iodine ablation therapy by providing first cancer surgery when indicated; and the disadvantages are the increased operative risks, such as the risk of permanent hypoparathyroidism and bilateral inferior laryngeal nerve injury; and permanent hypothyroidism. The extent of first operation is determined based on the presence of such clinical factors as a contralateral dominant thyroid nodule and thyroid dysfunction, as well as patient preference (5). Accordingly, a lobectomy may be an adequate first management option for most follicular variant of papillary thyroid carcinomas and minimally invasive follicular carcinoma, which constitutes a large proportion of thyroid cancers among ITTs (5).
The ATA suggests lobectomy for solitary indeterminate thyroid nodules, but suggests that a total thyroidectomy may be more appropriate if a cancer-specific mutation is detected (5). However, it has been accepted that mutations in RAS genes, the most frequent among ITTs, are usually related with low-risk malignancies, for which a lobectomy is frequently adequate. As such, actual “rule-in” tests may have a limited role in guiding the surgical extent of solitary ITTs. A total thyroidectomy could be planned as the initial procedure in patients in which a completion thyroidectomy would be recommended in order to give radioiodine treatment (5).
An uncertain ROM estimation in AUS/FLUS and FN/SFN patients results in suboptimal surgical planning, particularly among those who undergo unnecessary operations for benign nodules or those who need a second operation for a completion thyroidectomy, contributing to increased healthcare costs and morbidity.
Patients with cytologically TBSRTC category V nodules should generally be referred for surgery. In patients with smaller (<4 cm) TBSRTC category V nodules, lobectomy or total thyroidectomy are both reasonable techniques, while for patients with large nodules (>4 cm); with clinical or radiologic evidence of gross extrathyroidal extension; and with clinical or radiologic evidence of lymph node or distant metastases, or both, the preferred surgical technique is total thyroidectomy (5).
Two recent studies have shown that total thyroidectomy had no survival advantage over less-than-total thyroidectomy both in Korean and US papillary thyroid cancer patients (41,42). Therefore, the lobectomy should be preferred as the diagnostic surgery for the ITTs when stage I papillary carcinoma cannot be excluded.
In some studies it is stated that although the short-term costs of diagnostic thyroidectomy can be high, there are relatively few long-term costs as conclusively diagnosing a nodule as benign avoids the need for further follow-up by USG (43).
It should be considered that most thyroid cancers in the ITTs are low-risk cancers and if clinical cancer is excluded by ultrasound, as with active surveillance of low-risk thyroid cancer, even if the diagnosis of low-risk thyroid cancer is missed, it will not harm the patient. Thus, unnecessary diagnostic surgeries performed to benign and indeterminate tumors will be reduced.
Intraoperative frozen section (IFS) evaluation during diagnostic surgery for indeterminate nodules
The role of IFS for the evaluation of thyroid nodules has long been a subject of discussion. While advocates cite the ability of IFS to detect malignant lesions intraoperatively, allowing conversion to a total thyroidectomy and decreasing the requirement for a completion thyroidectomy in the future, there are others who claim that IFS uncommonly changes the management strategy and results in increased costs. The ATA 2015 updated guidelines state that IFS can sometimes confirm malignancy during a lobectomy in indeterminate nodules and give an opportunity for conversion to total thyroidectomy if indicated. At the same time, ATA states that IFS is most useful in classic papillary cancer, whereas its helpfulness is low in follicular variant of papillary thyroid carcinoma and follicular carcinoma (5). The evaluation of IFS for thyroid cancer can be difficult, and depends much on the experience of the pathologist. The problem of time wasted waiting in the operating room is a potentially underappreciated cost of IFS; however, in a prospective study, it has been shown that operation times are not significantly longer for IFS, taking on average 26 minutes (44). The time spent waiting for the IFS result can be used for hemostasis, closing the incision, and so on. Ultimately, limiting the usage of IFS to conditions in which conversion to a total thyroidectomy would actually be performed if a malignity were detected would result in a further decrease of healthcare resource usage. The utilization of IFS for patients with a cytologic diagnosis of SFM undergoing a diagnostic lobectomy allows for an immediate conversion to a total thyroidectomy, thereby reducing the number of completion thyroidectomies and leading to reduced healthcare resource usage. But as it stated previously conversion to a total thyroidectomy should not be recommended even if frozen section diagnosis revealed stage I papillary thyroid carcinoma (41,42).
The number of publications focused on FN, for which, theoretically, IFS gives the most helpful data, are low, and the outcomes are heterogeneous.
In an earlier retrospective study by Makay et al., it was found that the sensitivity of IFS was as low as 58%, and that the IFS results changed the first surgical strategy that was planned based on a preoperative FNAB and other diagnostic tools in only 16% of patients. The cost of IFS was lower than the cost of a completion thyroidectomy (45).
Healthcare resources and the management of indeterminate nodules
“The impact of each one on the other” & cost-effectiveness of diagnosis and management options of indeterminate nodules
In a recent meta-analysis, it was shown that thyroid cancers accounted for one-third of all operated nodules; that half of all operated nodules had an indeterminate cytology; and that two-thirds were found to be benign after a diagnostic thyroidectomy (20). According to a study by Valderrabano et al., the number of malign nodules was almost equal to the number of benign nodules that were resected due to an indeterminate cytology throughout 2016 in the United States, and that this overtreatment had resulted in some short- and long-term surgical complications, resulting in lifelong thyroid hormone replacement in most patients (10).
Molecular studies are not low-cost procedures, costing $3,000–$5,000 per test in 2015, depending on the specific testing procedure (46). There have been studies recommending molecular studies using the GEC as a cost-effective approach, primarily due to the decrease in diagnostic thyroidectomy numbers and surgical complications when the test results are negative (18). Proponents of molecular testing for indeterminate nodules have highlighted their cost-effectiveness, in that a benign result can avoid the need for thyroidectomy. There have been studies showing that Afirma® decreases costs, with the primary benefit being a decrease in the number of diagnostic thyroidectomies for indeterminate nodules that end up being benign (47). That said, the cost-modeling for Afirma® was limited to a short follow-up period that failed to take into account the long-term costs of surveillance for thyroid nodules in these studies. Balentine et al. reported diagnostic lobectomy to be both more effective and less costly than molecular testing (Afirma®) for the evaluation of ITTs after taking into account the cost of long-term (>5 years) follow-up, using a Markov decision-analytic model (43). In a single-center study in Canada, it was shown that ThyroSeq v3 molecular testing resulted in a significant reduction in the number of diagnostic thyroidectomies for ITTs (48). In a cost-comparison study between molecular tests and diagnostic lobectomy, Nicholson et al. reported ThyroSeq v3 to be the most cost-effective choice in indeterminate nodule diagnosis, resulting in a decrease of $24,131 per correct diagnosis when compared to diagnostic thyroidectomy (49). In a study using international guidelines as a framework, in particular the 2018 National Comprehensive Cancer Network Clinical Practice Guidelines for thyroid carcinoma, adapted for limited healthcare resource settings and low healthcare resource scenarios through the systematic removal of such elements as accessibility of thyroid-stimulating hormone, USG, radioactive iodine, the researchers showed that without thyroid hormone and/or calcium monitoring and replacement, Bethesda III and IV thyroid nodules should generally be followed up unless the lesion shows extremely suspicious ultrasonographic characteristics or growth over time (50).
In the performance of surgery in low- and middle-income countries there is potential for unwarranted damage and interruption to healthcare systems. For a long time, surgery in limited healthcare resource settings has been thought to be too logistically complicated and costly to address global disease. However, recent publications have supported the increase of the recognition and prioritization of surgery as a cost-effective instrument in global health. Researching long-term outcomes in limited healthcare resource settings is extremely difficult, as patients may not come back for follow-up and can be difficult to trace. In a study conducted in China, it was shown that the total annual cost of non-cancer thyroidectomies increased by 153% from USD 0.626 million in 2008 to USD 1.586 million in 2013. Among all thyroidectomies, the annual cancer rate was 41.6% in China, significantly lower than in developed countries. This difference has been associated with the belief among healthcare professionals in China, including thyroid surgeons and even endocrinologists, that surgery is the optimum approach to thyroid nodules, and cannot be replaced by other procedures, including thyroid FNAB (16). In the same study, the cost of ultrasound guided thyroid FNAB in the United States was estimated at USD 465.56, while in one of the largest hospitals in eastern China the average cost of thyroid FNAB was USD 45, including the cost of the cytopathology examination. As such, non-cancer patients would have been saved from unnecessary surgery by one or two FNABs with a total cost of no more than USD 89 (16).
In a recent meta-analysis it was shown that IFS is not cost-effective in cases of FN. IFS costs include those associated with the test itself, the technical and human resources needed for processing and interpretation, the operating room while waiting for the results to arrive, and the costs of the number of completion thyroidectomies that are required in false-negative cases (17). In another study, conducted by Bollig et al., it was shown that the routine use of IFS for SFM nodules resulted in cost savings of $474 per case (51).
In a recent meta-analysis in which data on indeterminate nodules were analyzed separately, a significant difference was noted in the resection rates in the Western and Asian contexts, and a more rapid switch to surgery in Western countries in contrast to the more strict selection of patients for surgery in Asia (5,52-56). Asian clinical guidelines suggest active surveillance for most TBSRTC categories, unlike in Western practice (5,54-56), and this difference in approach likely results in a higher resection rate and lower ROM of FN/SFN nodules in most Western series, whereas many patients with AUS/FLUS and FN/SFN nodules undergo active surveillance when presenting with benign clinical and ultrasonographic features in Asia. Suggestions of prompt diagnostic thyroidectomy for all FN/SFN nodules in the ATA and the National Comprehensive Cancer Network guidelines probably result in higher resection rates and relatively lower ROM in surgically treated FN/SFN nodules in Western patient series (5,53,57).
Conclusions
The optimal approach to the management of ITTs continues to develop. Clinical risk evaluation tools and algorithms must be incorporated into clinical practice to allow the personalized management of ITTs. The risk classification of indeterminate nodules has proven to be feasible based on such clinical factors as sex or nodule size; ultrasonographic features; and cytological characteristics such as the presence or absence of nuclear atypia, although it needs to be adapted to institutional results. Clinical and USG findings are important preoperative variables that are used to guide the initial recommendations. Today’s molecular testing procedures have evolved to resolve the diagnostic ambiguity related to ITTs, and initial studies into their use have been promising in the refinement preoperative risk stratification. That said, all kinds of molecular testing should be carried out, with care for the insufficiency of enough independent clinical validation studies and the unknown impact on mid- and long-term clinical results. Molecular testing may reduce the number of diagnostic surgeries, but the high cost makes it unavailable in many centers. Clinical presentation and patient preferences are still considerably influential in decision-making.
International guidelines for the management of thyroid nodules and cancers may not be practical in low healthcare resource scenarios. Failure to consider the limitations in access to healthcare resources can lead to inappropriate surgery and over-examination, or may even have life changing or ending consequences related to unexpected perioperative or postoperative complications.
Healthcare resources are highly influential in ITT management. In limited healthcare resource contexts, for TBSRTC category 3 and 4 nodules in particular, if there are no suspicious findings indicating thyroid cancer, diagnostic surgery is not preferred due to the potentially unnecessary surgery (thyroid hormone and/or calcium monitoring and replacement, postoperative complications, etc.) costs, as the majority of these nodules will be reported as benign. In Western countries, where there is greater access to healthcare resources, diagnostic surgery decisions are made faster than in Asian countries when indeterminate nodules are encountered. Thyroxine hormones may be cheaper in underdeveloped countries than in the West, but it should be noted that they can be quite expensive for those living in underdeveloped countries. Since SFM has a very high ROM, it is managed similar to thyroid cancer in the all healthcare resource settings.
From an economic perspective, when healthcare resources are limited, surgery should no longer be considered as a diagnostic procedure for ITTs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński and Maurizio Iacobone) for the series “Recent Challenges in the Management of Thyroid Tumors” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-44). The series “Recent Challenges in the Management of Thyroid Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reiners C, Wegscheider K, Schicha H, et al. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 2004;14:926-32. [Crossref] [PubMed]
- Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab 2006;91:3411-7. [Crossref] [PubMed]
- Werk EE, Vernon BM, Gonzalez JJ, et al. Cancer in thyroid nodules: a community hospital survey. Arch Intern Med 1984;144:474-6. [Crossref] [PubMed]
- Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol 2016;2:1023-9. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Hyeon J, Ahn S, Shin JH, et al. The prediction of malignant risk in the category “atypia of undetermined significance/follicular lesion of undetermined significance” of the Bethesda System for Reporting Thyroid Cytopathology using subcategorization and BRAF mutation results. Cancer Cytopathol 2014;122:368-76. [Crossref] [PubMed]
- Ferris RL, Baloch Z, Bernet V, et al. American Thyroid Association statement on surgical application of molecular profiling for thyroid nodules: current impact on perioperative decision making. Thyroid 2015;25:760-8. [Crossref] [PubMed]
- Paschke R, Cantara S, Crescenzi A, et al. European Thyroid Association guidelines regarding thyroid nodule molecular fine-needle aspiration cytology diagnostics. Eur Thyroid J 2017;6:115-29. [Crossref] [PubMed]
- Valderrabano P, McIver B. Evaluation and management of indeterminate thyroid nodules: the revolution of risk stratification beyond cytological diagnosis. Cancer Control 2017;24:1073274817729231 [Crossref] [PubMed]
- Hamming JF, Goslings BM, Van Steenis GJ, et al. The value of fine-needle aspiration biopsy in patients with nodular thyroid disease divided into groups of suspicion of malignant neoplasms on clinical grounds. Arch Intern Med 1990;150:113-6. [Crossref] [PubMed]
- Campanella P, Ianni F, Rota CA, et al. Quantification of cancer risk of each clinical and ultrasonographic suspicious feature of thyroid nodules: a systematic review and meta-analysis. Eur J Endocrinol 2014;170:R203-11. [Crossref] [PubMed]
- Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab 2014;99:1253-63. [Crossref] [PubMed]
- Vriens D, de Wilt JHW, van der Wilt GJ, et al. The role of [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: systematic review and meta-analysis of the literature. Cancer 2011;117:4582-94. [Crossref] [PubMed]
- Rago T, Santini F, Scutari M, et al. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92:2917-22. [Crossref] [PubMed]
- Liu XY, Zhu LJ, Cui D, et al. Annual financial impact of thyroidectomies for nodular thyroid disease in China. Asian Pac J Cancer Prev 2014;15:5921-6. [Crossref] [PubMed]
- Grisales J, Sanabria A. Utility of routine frozen section of thyroid nodules classified as follicular neoplasm: meta-analysis of diagnostic tests. Am J Clin Pathol 2020;153:210-20. [PubMed]
- Yip L, Farris C, Kabaker AS, et al. Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab 2012;97:1905-12. [Crossref] [PubMed]
- Cibas ES, Ali SZNCI Thyroid FNA State of the Science Conference. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 2009;132:658-65. [Crossref] [PubMed]
- Bongiovanni M, Spitale A, Faquin WC, et al. The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 2012;56:333-9. [Crossref] [PubMed]
- Valderrabano P, Leon ME, Centeno BA, et al. Institutional prevalence of malignancy of indeterminate thyroid cytology is necessary but insufficient to accurately interpret molecular marker tests. Eur J Endocrinol 2016;174:621-9. [Crossref] [PubMed]
- Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid 2011;21:243-51. [Crossref] [PubMed]
- Danese D, Sciacchitano S, Farsetti A, et al. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid 1998;8:15-21. [Crossref] [PubMed]
- Cesur M, Corapcioglu D, Bulut S, et al. Comparison of palpation-guided fine-needle aspiration biopsy to ultrasound-guided fine-needle aspiration biopsy in the evaluation of thyroid nodules. Thyroid 2006;16:555-61. [Crossref] [PubMed]
- Madubogwu CI, Ukah CO, Onyiaorah IV, et al. Cost effectiveness of fine needle aspiration cytology for breast masses. Orient J Med 2015;27:22-7.
- Reeves M, Patel R, Harmston C. Surgeon-performed ultrasound-guided fine needle aspiration of thyroid nodules is cost effective and efficient: evaluation of thyroid nodule assessment in a provincial New Zealand hospital. N Z Med J 2019;132:60-5. [PubMed]
- Janovsky CCPS, Bittencourt MS, Novais MAP, de , et al. Thyroid cancer burden and economic impact on the Brazilian public health system. Arch Endocrinol Metab 2018;62:537-44. [Crossref] [PubMed]
- Nikiforov YE. Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med 2011;135:569-77. [Crossref] [PubMed]
- Nikiforov YE, Carty SE, Chiosea SI, et al. Impact of the multi-gene ThyroSeq next-generation sequencing assay on cancer diagnosis in thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance cytology. Thyroid 2015;25:1217-23. [Crossref] [PubMed]
- Valderrabano P, Khazai L, Leon ME, et al. Evaluation of ThyroSeq v2 performance in thyroid nodules with indeterminate cytology. Endocr Relat Cancer 2017;24:127-36. [Crossref] [PubMed]
- Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 2012;367:705-15. [Crossref] [PubMed]
- Al-Qurayshi Z, Deniwar A, Thethi T, et al. Association of malignancy prevalence with test properties and performance of the gene expression classifier in indeterminate thyroid nodules. JAMA Otolaryngol Head Neck Surg 2017;143:403-8. [Crossref] [PubMed]
- Chen JC, Pace SC, Chen BA, et al. Yield of repeat fine-needle aspiration biopsy and rate of malignancy in patients with atypia or follicular lesion of undetermined significance: the impact of the Bethesda System for Reporting Thyroid Cytopathology. Surgery 2012;152:1037-44. [Crossref] [PubMed]
- Gharib H, Papini E, Paschke RDD, et al. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodule. J Endocrinol Invest 2010;33:1-50. [Crossref] [PubMed]
- Gharib H, Papini E, Paschke RDD, et al. AACE/AME/ETA Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodule. Endocr Pract 2010;16:468-75. [Crossref] [PubMed]
- NCCN. NCCN clinical practice guidelines in oncology (NCCN guidelines®): thyroid carcinoma, version 2. 2015. 2016.
- Choi SH, Baek JH, Lee JH, et al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results. Thyroid 2015;25:897-903. [Crossref] [PubMed]
- Patel SG, Yip L. Surgical intervention for indeterminate thyroid nodules. In: Roman SA, Sosa JA, Solórzano CC. editors. Management of thyroid nodules and differentiated thyroid cancer. Cham: Springer; 2017:147-62.
- The Japan Thyroid Association. Guidelines for clinical practice for the management of thyroid nodules in Japan 2013. Tokyo: Tokyo Nankodo Co., Ltd., 2013:1-277.
- Yi KH. The Revised 2016 Korean Thyroid Association Guidelines for thyroid nodules and cancers: differences from the 2015 American Thyroid Association Guidelines. Endocrinol Metab (Seoul) 2016;31:373-8. [Crossref] [PubMed]
- Welch HG, Doherty GM. Saving thyroids—overtreatment of small papillary cancers. N Engl J Med 2018;379:310-2. [Crossref] [PubMed]
- Zhang HS, Lee EK, Jung YS, et al. Total thyroidectomy’s association with survival in papillary thyroid cancers and the high proportion of total thyroidectomy in low-risk patients: analysis of Korean nationwide data. Surgery 2019;165:629-36. [Crossref] [PubMed]
- Balentine CJ, Vanness DJ, Schneider DF. Cost-effectiveness of lobectomy versus genetic testing (Afirma®) for indeterminate thyroid nodules: considering the costs of surveillance. Surgery 2018;163:88-96. [Crossref] [PubMed]
- Udelsman R, Westra WH, Donovan PI, et al. Randomized prospective evaluation of frozen-section analysis for follicular neoplasms of the thyroid. Ann Surg 2001;233:716-22. [Crossref] [PubMed]
- Makay O, Icoz G, Gurcu B, et al. The ongoing debate in thyroid surgery: should frozen section analysis be omitted? Endocr J 2007;54:385-90. [Crossref] [PubMed]
- Nishino M. Molecular cytopathology for thyroid nodules: a review of methodology and test performance. Cancer Cytopathol 2016;124:14-27. [Crossref] [PubMed]
- Wu JX, Lam R, Levin M, et al. Effect of malignancy rates on cost-effectiveness of routine gene expression classifier testing for indeterminate thyroid nodules. Surgery 2016;159:118-26. [Crossref] [PubMed]
- Chen T, Gilfix B, Rivera JA, et al. The role of the ThyroSeq v3 molecular test in the surgical management of thyroid nodules in the Canadian public healthcare setting. Thyroid 2020;30:1280-7. [Crossref] [PubMed]
- Nicholson KJ, Roberts MS, McCoy KL, et al. Molecular testing versus diagnostic lobectomy in Bethesda III/IV thyroid nodules: a cost-effectiveness analysis. Thyroid 2019;29:1237-43. [Crossref] [PubMed]
- Zafereo M, Yu J, Onakoya PA, et al. African Head and Neck Society Clinical Practice guidelines for thyroid nodules and cancer in developing countries and limited resource settings. Head Neck 2020;42:1746-56. [Crossref] [PubMed]
- Bollig CA, Gilley D, Lesko D, et al. Economic impact of frozen section for thyroid nodules with "suspicious for malignancy" cytology. Otolaryngol Head Neck Surg 2018;158:257-64. [Crossref] [PubMed]
- Kakudo K, Kameyama K, Miyauchi A, et al. Introducing the reporting system for thyroid fine-needle aspiration cytology according to the new guidelines of the Japan Thyroid Association. Endocr J 2014;61:539-52. [Crossref] [PubMed]
- Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81:1-122. [Crossref] [PubMed]
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract 2016;22:622-39. [Crossref] [PubMed]
- Kakudo K, Higuchi M, Hirokawa M, et al. Thyroid FNA cytology in Asian practice—active surveillance for indeterminate thyroid nodules reduces overtreatment of thyroid carcinomas. Cytopathology 2017;28:455-66. [Crossref] [PubMed]
- Haddad R. Practice guidelines in oncology—thyroid carcinoma v.2.2017. 2017. Available online: http://www.nccn.org/profe ssion als/physi cian_gls/PDF/thyro id.pdf (accessed June 13, 2018).
Cite this article as: Alci E, Makay Ö. Impact of healthcare resources on management of indeterminate thyroid tumors. Ann Thyroid 2021;6:3.


