
Current surgical management of follicular thyroid carcinoma
Background
Changes in the histological definition of follicular thyroid carcinoma (FTC)—from American Registry of Pathology (ARP) 2016 to to World Health Organization (WHO) 2017
FTC is a malignant epithelial tumor of the thyroid gland with follicular differentiation, which is characterized by the absence of the nuclear features typical of papillary thyroid carcinoma (PTC). In 2016, the ARP divided FTC into two main types: minimally invasive FTC and widely invasive FTC (1). The majority (approximately two thirds) of FTC belong to the minimally invasive type (2). This subtype comprises “minimally invasive FTC with capsular invasion (not obvious invasion)”, “minimally invasive FTC with limited vascular invasion (< four vessels)” and “minimally invasive FTC with extensive vascular invasion (≥ four vessels)”. The current 2017 World Health Organization (WHO) classification of tumors of endocrine organs, however, describes three histological subtypes (3): minimally invasive FTC with capsular invasion only (miFTC), encapsulated angioinvasive FTC [eaFTC with limited vascular invasion (< four vessels) and with extensive vascular invasion (≥ four vessels)] and widely invasive FTC (wiFTC). In contrast to ARP, the WHO 2017 definition emphasizes the distinction between minimally invasive FTC with capsular invasion but absent vascular invasion and the encapsulated variant of FTC with angioinvasion.
Epidemiology of FTC
FTC accounts for 10 to 15 per cent of differentiated thyroid carcinomas, following PTC (4,5). The majority of patients diagnosed with FTC is female (ratio approximately 2.5:1), with an age peak in the fifth and sixth decade of life (6-9). Locoregional lymphatic spread is rare in FTC (1 to 7 per cent) (10-12), whereas hematogenous metastases to the lung and bone are observed more frequently (6 to 20 per cent) (6,13).
Dilemma of the adaption of the surgical strategy to a “follicular neoplasm”
In comparison to PTC, which—facilitated by fine-needle aspiration cytology (FNAC) and additional molecular genetic analysis (e.g., BRAFV600E mutation indicating PTC)—can be diagnosed prior to an operation, follicular thyroid lesions present a challenge to treating surgeons. There are currently no reliable means to preoperatively distinguish between follicular adenoma and the three variants of FTC, as described in the 2017 WHO classification. Therefore, the surgical management cannot precisely be adapted to the underlying entity—neither pre- nor intraoperatively. Current guidelines of international expert associations as well as numerous studies published in the literature address this surgical dilemma of follicular thyroid neoplasms (14). So far, preoperative decision-making can be based almost only upon patient stratification depending on clinical risk factors (advanced patient age, tumor size, presence of metastases). The recent changes of the WHO 2017 classification of endocrine neoplasms implicate that an optimized resection strategy, according to the subtypes miFTC, eaFTC and wiFTC, could avoid unnecessary radical resection with associated higher operative risk.
Diagnostic workup of follicular lesions prior to surgery—limited by existing methods
FTCs usually appear as cold thyroid nodules on radionuclide scan (7). Yet, the positive predictive value of a “cold nodule” for FTC presence is low. Sonography can be helpful in distinguishing between FTC and follicular adenoma of the thyroid gland: larger lesion size, absence of a sonographic halo, hypoechoic appearance and absence of cystic changes favor the diagnosis of follicular carcinoma (15). However, compared to PTC, typical sonographic features are frequently absent, especially in case of miFTC and eaFTC. A standardized analysis of suspicious thyroid nodules, e.g., using the European Thyroid Association (ETA) Guidelines for Ultrasound Malignancy Risk Stratification (“Eu-TIRADS”) (16) or the American Thyroid Association (ATA) Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer (17)—facilitates the selection of patients with a potential benefit from undergoing FNAC. Yet, FNAC analysis frequently leads to indeterminate results [Bethesda category: III “atypia of undetermined significance or follicular lesion of undetermined significance”, IV “follicular neoplasm or suspicious for follicular neoplasm” (18)]. Due to the methodological limitation of FNAC (and similarly intraoperative frozen section analysis), which does not allow for a conclusive evaluation capsular and vascular invasion (Figure 1), the diagnosis of FTC cannot unambiguously be established (19,20). Accordingly, only one third of patients with thyroid nodules of Bethesda category III actually harbor malignant diagnoses (21). To exclude potential malignancy in thyroid nodules with uncertain results in FNAC, the evaluation of mRNA expression using microarray gene expression classifier (GEC) also has diagnostic value (22). Drawbacks of the method—in addition to cost—are that long-term outcome data of patients excluded from surgery, due to negative results in GEC, are not yet available (23,24). Furthermore, the predictive value depends on the prevalence of malignancy in the analyzed cohorts. The ETA recommends the additional molecular evaluation of BRAF, RET/PTC, PAX8-PPARγ and RAS in suspicious thyroid nodules (23). Still, molecular analysis only cannot serve to establish an indication for surgery in case of follicular thyroid neoplasms, due to the ambiguous results regarding potential malignancy. Instead, all preoperatively available results including clinical features, sonographic and radionuclide imaging as well as cytological and molecular genetic findings must be interpreted in context with each other to establish a surgical treatment strategy. Moreover, the value of intraoperative frozen sections as an additional diagnostic tool during surgery, is controversially discussed for FTC. Only the histopathological evaluation of formalin-fixed, paraffin-embedded tissue sections allows the doubtless diagnosis of FTC and the characterization of its subtypes (19,25,26). However, if vascular invasion can be diagnosed in frozen sections, it is usually associated with widely invasive FTC, directing the operation towards a more radical procedure. The German Association of Endocrine Surgeons (CAEK) acknowledged the importance of frozen section analysis for the exclusion of other malignant diagnoses in preoperatively diagnosed follicular neoplasms (7,27-29).

Prognostic factors for FTC: clinical and histological criteria
In comparison to PTC, FTC is associated with a higher tumor-specific mortality rate (30). Yet, within the group of FTCs there is a large variety concerning long-term prognosis: whereas long-term survival rates in patients with metastatic FTC range from 31 per cent to 43 per cent (6), for miFTC without risk factors, an excellent outcome was demonstrated, with survival rates being similar to the average US population (31). Manifold studies, including uni- and multivariable analyses, were performed, to analyze risk factors for an impaired outcome in FTC. Prognostic factors, which had been detected in previous multivariable studies before 2008, were confirmed in the last decade (32) (Tables 1-3). These include distant metastasis, tumor size and extrathyroidal extension (32) (Table 1). Recent multivariable analyses, which focused on disease-free survival, identified widely invasive histology, tumor size, distant metastasis and extrathyroidal extension as defining risk factors (2,38,39) (Table 1). Advanced patient age, angioinvasion and initial lymph node metastasis were described as relevant for the development of distant metastases during postoperative follow-up (35). Overall survival was significantly influenced by patient age, tumor size, surgical margins, distant metastasis and locoregional recurrence (40). Recognizing the different tumor biology of FTC subgroups, different authors performed separate analyses of prognostic factors for FTCs with minimally invasive and widely invasive histology (Tables 2,3). In case of minimally invasive FTC, disease-free survival was significantly influenced by angioinvasion, tumor size and advanced patient age (37,43) (Table 2). For widely invasive FTC, tumor size and distant metastasis were relevant for disease-free survival (37,46). Generally, widely invasive FTC is associated with a significantly worse carcinoma-specific survival and disease-free survival than minimally invasive FTC (2,35). Whereas carcinoma-specific survival (10 years) was shown to be 93.5 per cent for minimally invasive FTC (ARP definition, comprising miFTC and eaFTC), for widely invasive FTC, 53.6 per cent were registered (2). Yet, most of the abovementioned analyses were primarily based on the histological subgroups according to the ARP definition (1). The subgroup eaFTC, added by the WHO in 2017, is not yet well represented as an independent category of analysis, nor as a potential prognostic factor for FTC itself.
Table 1
| Authors | Year | Prognostic factor | Endpoint | Patients included | Median/mean follow-up (months) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced patient age | Male sex | Female sex | Tumor size | Postoperative histology | Metastasis | Locoregional recurrence | Genetic disorder | Survival | Metastasis | |||||||||||||||||||||||
| Widely invasive histology | Angio invasion |
Capsular invasion | Multifocality | Extrathyroidal extension | Surgical margins | LNM | DM | Mutational burden | Driver gene mutation for FTC (33) | Driver genes for cancer (34) | EIF1AX mutation | TERT promoter mutation | H/N/K-RAS mutation | DFS | OS | CSD | CSS | DMFS | LNM | DM | ||||||||||||
| Alfalah et al. (10) | 2008 | – | – | – | + | – | Xpr | 70 | Mean: 52 | |||||||||||||||||||||||
| Asari et al. (2) | 2009 | – | – | – | – | + | + | Xpr | 207 | 86 | ||||||||||||||||||||||
| + | – | – | + | + | – | Xpr | 207 | 86 | ||||||||||||||||||||||||
| + | – | ⊕ | ⊕ | + | + | ⊕ | X | 207 | 86 | |||||||||||||||||||||||
| O’Neill et al. (35) | 2011 | ⊕ | – | – | – | ⊕ | ⊕ | Xf | 124 | 40 | ||||||||||||||||||||||
| Sugino et al. (36) | 2011 | + | – | – | – | + | – | + | + | Xpr | 134 | 150 | ||||||||||||||||||||
| + | – | – | – | – | – | – | – | Xf | 121 | 150 | ||||||||||||||||||||||
| ⊕ | + | + | – | ⊕ | X | 134 | 150 | |||||||||||||||||||||||||
| ⊕ | ⊕ | ⊕ | – | X | 134 | 150 | ||||||||||||||||||||||||||
| Kim et al. (37) | 2014 | + | – | + | ⊕ | – | + | ⊕ | X | 204 | 55 | |||||||||||||||||||||
| Podda et al. (38) | 2015 | ⊕ | X | 71 | Mean: 113/125 | |||||||||||||||||||||||||||
| Rios et al. (39) | 2015 | + | + | + | + | + | ⊕ | X | 66 | Mean: 99 | ||||||||||||||||||||||
| Su et al. (40) | 2019 | ⊕ | – | – | ⊕ | – | + | ⊕ | + | ⊕ | ⊕ | X | 204 | 77 | ||||||||||||||||||
| + | ⊕ | – | – | – | + | ⊕ | + | ⊕ | ⊕ | X | 204 | 77 | ||||||||||||||||||||
| Nicolson et al. (33) | 2018 | + | – | – | – | + | + | ⊕ | + | + | X | 39 | 69 | |||||||||||||||||||
| Duan et al. (41) | 2019 | – | – | – | – | + | + | + | + | – | X | 51 | 56 | |||||||||||||||||||
Factors analyzed, but not confirmed as prognostically relevant, are marked with “–”. Factors confirmed in univariable analysis are marked with “+”. Factors proven as significant in multivariable analysis are marked with “⊕”. Analyzed endpoints are marked with “X”. The endpoint “metastasis” includes “Xpr” (diagnosis preoperatively, or immediately after primary surgery) and “Xf ” (diagnosis during follow-up). DFS, disease-free survival; OS, overall survival; CSD, cause-specific death; CSS, cause-specific survival; DMFS, distant metastasis-free survival; LNM, lymph node metastasis; DM, distant metastasis.
Table 2
| Authors | Year | Prognostic factor | Endpoint | Patients included | Median/mean follow-up (months) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced patient age | Male sex | Female sex | Tumor size | Postoperative histology | Metastasis | RNA H19 expression | Survival | DM | ||||||||||||
| Angioinvasion | Capsular invasion | Multifocality | LNM | DM | DFS | CSD | CSS | DMFS | ||||||||||||
| Asari et al. (2) | 2009 | + | – | + | – | X | 127 | 86 | ||||||||||||
| – | – | – | – | + | X | 127 | 86 | |||||||||||||
| Sugino et al. (42) | 2012 | + | – | – | – | – | + | – | Xpr | 251 | 86 | |||||||||
| + | – | – | + | – | – | – | Xf | 244 | 86 | |||||||||||
| ⊕ | + | – | – | + | X | 251 | 86 | |||||||||||||
| ⊕ | + | – | – | X | 251 | 86 | ||||||||||||||
| Ito et al. (43) | 2013 | ⊕ | – | ⊕ | ⊕ | – | – | X | 285 | Mean: 117 | ||||||||||
| – | – | ⊕ | ⊕ | – | – | ⊕ | X | 292 | Mean: 117 | |||||||||||
| Kim et al. (37) | 2014 | – | – | – | ⊕ | – | ⊕ | X | 165 | 55 | ||||||||||
| Stenson et al. (44) | 2016 | + | ⊕ | – | + | + | X | 58 | 140 | |||||||||||
| Dai et al. (45) | 2019 | ⊕ | – | + | ⊕ | ⊕ | – | – | + | Xf | 186 | 109 | ||||||||
Factors analyzed, but not confirmed as prognostically relevant, are marked with “–”. Factors confirmed in univariable analysis are marked with “+”. Factors proven as significant in multivariable analysis are marked with “⊕”. Analyzed endpoints are marked with “X”. The endpoint “metastasis” includes “Xpr” (diagnosis preoperatively, or immediately after primary surgery) and “Xf” (diagnosis during follow-up). LNM, lymph node metastasis; DM, distant metastasis; DFS, disease-free survival; CSD, cause-specific death; CSS, cause-specific survival; DMFS, distant metastasis-free survival.
Table 3
| Authors | Year | Prognostic factor | Endpoint | Patients included | Median/mean follow-up (months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Advanced patient age | Male sex | Female sex | Tumor size | Postoperative histology | Metastasis | Survival | |||||||||
| Angioinvasion | Multifocality | LNM | DM | DFS | CSS | ||||||||||
| Asari et al. (2) | 2009 | + | + | – | – | – | X | 80 | 86 | ||||||
| + | – | ⊕ | – | – | ⊕ | X | 80 | 86 | |||||||
| Ito et al. (46) | 2013 | – | – | – | ⊕ | X | 70 | Mean: 117 | |||||||
| – | – | – | – | ⊕ | X | 79 | Mean: 117 | ||||||||
| Kim et al. (37) | 2014 | – | – | – | – | – | – | ⊕ | X | 39 | 55 | ||||
Factors analyzed, but not confirmed as prognostically relevant, are marked with “–”. Factors confirmed in univariable analysis are marked with “+”. Factors proven as significant in multivariable analysis are marked with “⊕”. Analyzed endpoints are marked with “X”. LNM, lymph node metastasis; DM, distant metastasis; DFS, disease-free survival; CSS, cause-specific survival.
An analysis of 39 cases of FTC, which was published in 2018, showed that—compared to miFTC and eaFTC—wiFTC had significantly higher American Joint Committee on Cancer (AJCC) stages at time of surgery and was associated with a higher likelihood of recurrence and of being cause of death (33). Though not significant, 10-year disease-specific survival was impaired in cases of eaFTC and wiFTC, but not in miFTC (33). Less aggressive therapy was already proposed for so-called low-risk FTCs in current expert recommendations (Table 4), although only further large-scale follow-up analyses will be able to prove adequateness of the surgical treatment options for the particular subgroups of FTC in the future. As histological FTC subtypes and clinical risk factors were shown to be associated with dissimilar prognosis and course of disease, different surgical treatment strategies are required in turn, once the diagnosis on the specific subtype is available. Addressing the issue, international expert societies have published recommendations based on FTC histology and clinical risk factors (Table 4).
Table 4
| Recommendations | Entity | Society recommendations | ||||
|---|---|---|---|---|---|---|
| WHO Definition 2017, (3) | German Association of Endocrine Surgeons (CAEK) 2013, (7) | European Society of Endocrine Surgeons (ESES) 2014, (47) | British Thyroid Association (BTA) 2014, (48) | American Thyroid Association (ATA) 2015, (17) | ||
| Recommended thyroid resection strategy | Minimally invasive FTC, capsular invasion only (miFTC) | Hemithyroidectomy or thyroidectomy (in cases of multinodular disease) | Hemithyroidectomy or thyroidectomy (if following risk factors are present: ≥45 years at presentation, tumor size ≥40 mm, vascular invasion present, positive lymph nodes, positive distant metastases) | Hemithyroidectomy or thyroidectomy (if following risk factors are present: age >45, widely invasive histology, lymph node/distant metastases, angioinvasion, tumor size >4 cm) | Lobectomy (for low- to intermediate-risk: unifocal tumors <4 cm, no evidence of extrathyroidal extension or lymph node metastases) or near-total/total thyroidectomy (if overall strategy includes RAI therapy. Tumors between 1 and 4 cm: bilateral procedure recommended if risk factors are present: older age (>45 years), contralateral thyroid nodules, a personal history of radiation therapy to the head and neck, familial differentiated thyroid carcinoma (because of plans for RAI therapy or to facilitate follow-up strategies or address suspicions of bilateral disease)) | |
| Encapsulated angioinvasive FTC (eaFTC) | Thyroidectomy | Thyroidectomy | Thyroidectomy | Near-total/total thyroidectomy (high risk: >4 vessels with angioinvasion, justifies a more aggressive approach) | ||
| Widely invasive FTC—grossly invasive (wiFTC) | Thyroidectomy | Thyroidectomy | Thyroidectomy | Near-total/total thyroidectomy | ||
| Recommended lymph node resection strategy | All FTC types | No prophylactic lymph node dissection; therapeutic lymph node dissection in case of evidence of lymph node metastasis before or during the operation (extent of resection depending on localization of proven lymph node metastases) | No prophylactic lymph node dissection; therapeutic lymph node dissection in case of evidence of lymph node metastasis before or during the operation | No prophylactic lymph node dissection; therapeutic lymph node dissection in case of evidence of lymph node metastasis before or during the operation (if there is preoperative or intraoperative suspicion of nodal disease, FNAC or frozen section should be performed prior to therapeutic node dissection) | No prophylactic lymph node dissection; therapeutic lymph node dissection in case of evidence of lymph node metastasis before or during the operation (therapeutic central-compartment (level VI) neck dissection for patients with clinically involved central nodes should accompany total thyroidectomy. Therapeutic lateral neck compartmental lymph node dissection should be performed for patients with biopsy-proven metastatic lateral cervical lymphadenopathy) | |
| Recommended postoperative radioiodine (RAI) treatment | All FTC types | RAI recommended for FTC with angioinvasion (independent from number of vessels affected) and for wiFTC | RAI indicated for elderly patients (>45 years), large tumor size (>40 mm), extensive vascular invasion, presence of distant synchronous or metachronous metastasis, positive nodes and if recurrence is noted in follow-up | No indication for RAI if all criteria are met: follicular carcinoma, tumor <1 cm, unifocal or multifocal, minimally invasive without angioinvasion, no invasion of thyroid capsule (extra thyroidal extension) | RAI recommended for high-risk DTC (e.g., eaFTC with >4 vessels affected), if the primary thyroid carcinoma is >4 cm, if there is gross extrathyroidal extension, or regional or distant metastasis, older age (>45 years), contralateral thyroid nodules, a personal history of radiation therapy to the head and neck or familial differentiated thyroid carcinoma | |
| Definite indication if one criterion is met: tumor >4 cm, any tumor size with gross extra thyroidal extension, distant metastases present | ||||||
| Uncertain indication if one of following criteria is met: large tumor size, extra-thyroidal extension, widely invasive histology, multiple lymph node involvement, large size of involved lymph nodes, high ratio of positive to negative nodes, extracapsular nodal involvement | ||||||
Genetic background—a potential help for preoperative diagnosis and risk assessment?
PTC can be confirmed preoperatively by the detection of a BRAFV600E mutation in fine-needle aspirates (49). Furthermore, specific RET/PTC rearrangements indicate PTC (50). For FTC, mutations of RAS (NRAS, HRAS, KRAS) and/or a PAX8-PPARγ gene fusion are typical (33,49,51). Yet, their detection in fine-needle aspirates only gives a hint for the presence of malignancy, since benign follicular thyroid adenoma can harbor the very same molecular alterations (52,53). A potential adenoma-carcinoma sequence is postulated for FTCs, especially for RAS-mutant tumors (51). RAS mutations can be associated with FTCs with a variable range of invasiveness (51). The PAX8-PPARγ gene fusion described relevant in FTC with “overtly invasive histology” (i.e., tumors confined to the thyroid gland and harboring at least three, typically more than five areas of capsular and vascular invasion) (51,52). Coexistence of HRAS, NRAS or KRAS mutations and a TERT promoter mutation were associated with advanced disease at time of diagnosis (41). Depending on the analyzed patient cohort, RAS mutations are detected in 40–50 per cent of FTC, whereas PAX8-PPARG fusion is present in 25–63 per cent (51,54,55). Moreover, the following genes were described to potentially carry driver mutations for FTC: BRAF, BRIP1, CNOT1, DICER1, EIF1AX, EZH1, IDH1, IGF2BP3, KDM5C, KMT2C, MAP4K3, NF1, PTEN, PI3KCA, SOS1, SPOP, STAG2, TCF12, TP53 and TSHR (33,41,54,56). Microsatellite instability (MSI) as a result of DNA mismatch repair (MMR) inactivation was described to be present in 2.5 per cent of FTC cases, and nearly absent in other malignant tumors deriving from the thyroid (57). The coincidence of MSI and loss of heterozygosity (LOH) in several, different loci (= “overall frequency of allelic loss”, OFAL) was described as a biomarker to distinguish FTC and follicular thyroid adenoma from PTC and nodular goiter (58). Mutational burden—a biomarker measuring the total number of present mutations—was identified as a predictor for mortality and recurrence, independent from the histological subgroup of FTC (33). Nowadays, mRNA expression analyses of multiple, thyroid- or tumor-associated molecular genetic alterations by microarray GEC (based on Affymetrix Human Exon 1.0 GeneChip) (22) are available, yet without reimbursement of cost in most health insurance systems and uncertain value concerning the treatment of patients with suspicious follicular thyroid nodules (23). Since up to now, no molecular marker has been identified, which can reliably be used for preoperative diagnosis of FTC, the surgical strategy has to be established with clinical risk factors as a basis. However, in the last 10 years, in addition to the “classical” risk factors for a poorer outcome of FTC (based on clinical and histopathological criteria), novel molecular predictors are also taken into consideration by different study groups (Tables 1,2).
Surgical treatment of FTC
Facing the difficulties of preoperative diagnosis, there is general consensus about how to surgically approach patients with follicular lesions. Basically, all patients diagnosed with solitary thyroid nodules with an indeterminate result in FNAC [Bethesda category III–IV (18)] and/or the clinical suspicion of malignancy (e.g., tumor size, accelerated growth, ultrasound or other imaging indicators, pathological lymph node status or distant lesions suspicious of metastases), should receive comprehensive information about the therapeutic options. This includes the well-known limitations, e.g., to intraoperatively distinguish minimally invasive, angioinvasive and widely invasive subtypes (7). Before surgery, patients should be made aware that the decision whether to perform lobectomy or thyroidectomy can be made intraoperatively by the surgeon. Depending on the histopathological result, in case of an initial lobectomy, secondary surgery may be necessary to perform a completion thyroidectomy.
Thyroid resection
Both the BTA and the ATA published different recommendations for surgical treatment of FTC based on the risk factor “tumor size” in combination with clinical and histological predictors. For FTCs with a diameter >4 cm, a total thyroidectomy is proposed (Table 4) (17,48). For FTCs with a tumor diameter >1 and ≤4 cm, hemithyroidectomy is considered a sufficient treatment, if the following risk factors (BTA) are absent: age >45 years, widely invasive histology (as judged visually in the operative situs, or by frozen section), lymph node/distant metastases, angioinvasion (48). According to the ATA, age (>45 years), contralateral thyroid nodules, personal history of head and neck radiotherapy and familial differentiated thyroid carcinoma are factors that should favor thyroidectomy, either because of plans for radioiodine (RAI) therapy or to facilitate follow-up strategies (17). The assessment of ATA risk factors is of course easier to perform preoperatively, while BTA risk factors may require an expert frozen section assessment. A consensus statement by the European Society of Endocrine Surgeons (ESES) recommends (completion) thyroidectomy followed by RAI treatment in minimally invasive FTC only in case of tumor size ≥4 cm, patients ≥45 years of age and in case of vascular invasion and/or nodal/distant metastases (47).
In contrast to the above-mentioned approaches, the practice guidelines of the German Society of Endocrine Surgery (CAEK) for the surgical treatment of malignant thyroid tumors are primarily based on histological patterns: for minimally invasive FTC with capsular invasion only, regardless of tumor size, hemithyroidectomy is recommended (7). Also, the ESES considered hemithyroidectomy a sufficient treatment for minimally invasive FTC (in absence of risk factors: <45 years at presentation, tumor size <4 cm and no angioinvasion or nodal/distant metastasis) (47). Yet, multinodular disease can be a reason for total thyroidectomy in patients with minimally invasive FTC with capsular invasion only (7). The CAEK recommends total thyroidectomy (including postoperative RAI treatment) in patients harboring FTC with proven angioinvasion as well as for widely invasive FTC (7,17,48). In case of an initially performed lobectomy, a completion thyroidectomy should be performed in these patients, to facilitate RAI treatment (7,17). Minimally invasive FTC with capsular invasion only, due to a low risk of metastasis, does not require completion thyroidectomy, independent from tumor size (Table 4) (7). In case of widely invasive FTC with extrathyroidal spread, the presence of invasion of the infrahyoid muscles or into perithyroidal veins and sometimes internal jugular veins can be identified preoperatively or intraoperatively. The veins can be filled with large tumor thrombi, sometimes extending into the upper chest; the surgical resection is performed with palliative intention in these difficult situations.
A key factor for complete and successful locoregional tumor resection (local R0) is the timely recognition of the advanced tumor growth. Multivisceral en-bloc resections of the thyroid, infiltrated vessels and strap muscles can prevent invasion of the esophago-tracheal axis while distant metastases can be controlled with adjuvant radio-iodine treatment or targeted therapy concepts (Figure 2).
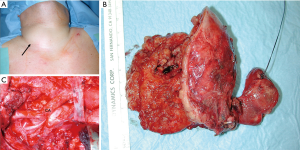
Lymph node dissection
Recognizing the fact that the driving prognostic factor for FTC is the presence of distant metastases instead of lymph node metastases, current recommendations of expert societies emphasize the limited value of a prophylactic central lymph node dissection, while therapeutic lymph node dissection is indicated (Table 4) (7,17,47,48). The BTA recommends frozen section analysis to evaluate the necessity of lymphadenectomy (48). According to the German CAEK, central lymphadenectomy may be performed in cases with intraoperative verification of thyroid carcinoma due to the fact that the differentiation between follicular variant of PTC and FTC is incorrect in many cases of frozen section (7). However, the new “low-risk” classification of the follicular variant of PTCs and the equally excellent prognosis of the encapsulated follicular variant of PTCs may prompt a revision of this recommendation, which is currently defined by an interdisciplinary expert panel. The ATA argues for a central and lateral lymph node dissection, in case of biopsy-proven lymph node metastases (17).
Conclusions
In the majority of cases, preoperative radiological imaging, FNAC and molecular analysis cannot unambiguously distinguish between benign and malignant follicular lesions. Yet, the malignant potential of a follicular thyroid lesions can be assessed by stratifying the patient according to clinical risk factors (presence of metastases, advanced patient age, tumor size). Based on verified risk factors, an individual approach should be chosen, following a stepwise, escalating surgical approach with restricted primary resection (hemithyroidectomy) and—if necessary—completion surgery based on the definitive histopathology.
Acknowledgments
The authors would like to thank Dr. Arno Schad, Institute of Pathology, University Medical Center Mainz, Germany, for the contribution of the histological images.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński and Maurizio Iacobone) for the series “Recent Challenges in the Management of Thyroid Tumors” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-28). The series “Recent Challenges in the Management of Thyroid Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosai J, DeLellis R, Carcangiu M, et al. Tumors of the Thyroid and Parathyroid Glands. Arlington, VA: American Registry of Pathology, 2016.
- Asari R, Koperek O, Scheuba C, et al. Follicular thyroid carcinoma in an iodine-replete endemic goiter region: a prospectively collected, retrospectively analyzed clinical trial. Ann Surg 2009;249:1023-31. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Klöppel G, et al. WHO classification of tumours of endocrine organs. Lyon: IARC,2017:65-143.
- Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 2013;13:184-99. [Crossref] [PubMed]
- Dralle H, Machens A, Basa J, et al. Follicular cell-derived thyroid cancer. Nat Rev Dis Primers 2015;1:15077. [Crossref] [PubMed]
- Parameswaran R, Shulin Hu J, Min En N, et al. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann R Coll Surg Engl 2017;99:151-4. [Crossref] [PubMed]
- Dralle H, Musholt TJ, Schabram J, et al. German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg 2013;398:347-75. [Crossref] [PubMed]
- Aschebrook-Kilfoy B, Grogan RH, Ward MH, et al. Follicular thyroid cancer incidence patterns in the United States, 1980-2009. Thyroid 2013;23:1015-21. [Crossref] [PubMed]
- Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980-2005. Cancer Epidemiol Biomarkers Prev 2009;18:784-91. [Crossref] [PubMed]
- Alfalah H, Cranshaw I, Jany T, et al. Risk factors for lateral cervical lymph node involvement in follicular thyroid carcinoma. World J Surg 2008;32:2623-6. [Crossref] [PubMed]
- Ito Y, Miyauchi A. Lateral and mediastinal lymph node dissection in differentiated thyroid carcinoma: indications, benefits, and risks. World J Surg 2007;31:905-15. [Crossref] [PubMed]
- Passler C, Scheuba C, Asari R, et al. Importance of tumour size in papillary and follicular thyroid cancer. Br J Surg 2005;92:184-9. [Crossref] [PubMed]
- Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer 2005;103:2269-73. [Crossref] [PubMed]
- Staubitz JI, Musholt PB, Musholt TJ. The surgical dilemma of primary surgery for follicular thyroid neoplasms. Best Pract Res Clin Endocrinol Metab 2019;33:101292. [Crossref] [PubMed]
- Sillery JC, Reading CC, Charboneau JW, et al. Thyroid Follicular Carcinoma: Sonographic Features of 50 Cases. AJR Am J Roentgenol 2010;194:44-54. [Crossref] [PubMed]
- Russ G, Bonnema SJ, Erdogan MF, et al. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur Thyroid J 2017;6:225-37. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017;27:1341-6. [Crossref] [PubMed]
- Rosai J. Handling of thyroid follicular patterned lesions. Endocr Pathol 2005;16:279-83. [Crossref] [PubMed]
- LiVolsi VA. Baloch ZW. Use and abuse of frozen section in the diagnosis of follicular thyroid lesions. Endocr Pathol 2005;16:285-93. [Crossref] [PubMed]
- Mileva M, Stoilovska B, Jovanovska A, et al. Thyroid cancer detection rate and associated risk factors in patients with thyroid nodules classified as Bethesda category III. Radiol Oncol 2018;52:370-6. [Crossref] [PubMed]
- Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 2012;367:705-15. [Crossref] [PubMed]
- Paschke R, Cantara S, Crescenzi A, et al. European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. Eur Thyroid J 2017;6:115-29. [Crossref] [PubMed]
- Marti JL, Avadhani V, Donatelli LA, et al. Wide Inter-institutional Variation in Performance of a Molecular Classifier for Indeterminate Thyroid Nodules. Ann Surg Oncol 2015;22:3996-4001. [Crossref] [PubMed]
- Schmid KW, Musholt T, Führer D. Knoten in der Schilddrüse – Histologie und Zytologie. Nuklearmediziner 2016;39:191-8. [Crossref]
- Schmid KW, Farid NR. How to define follicular thyroid carcinoma? Virchows Arch 2006;448:385-93. [Crossref] [PubMed]
- Udelsman R, Westra WH, Donovan PI, et al. Randomized prospective evaluation of frozen-section analysis for follicular neoplasms of the thyroid. Ann Surg 2001;233:716-22. [Crossref] [PubMed]
- Lin HS, Komisar A, Opher E, et al. Follicular variant of papillary carcinoma: the diagnostic limitations of preoperative fine-needle aspiration and intraoperative frozen section evaluation. Laryngoscope 2000;110:1431-6. [Crossref] [PubMed]
- Baloch Z. Role of repeat fine-needle aspiration biopsy (FNAB) in the management of thyroid nodules. Diagn Cytopathol 2003;29:203-6. [Crossref] [PubMed]
- Liu Z, Zeng W, Huang L, et al. Prognosis of FTC compared to PTC and FVPTC: findings based on SEER database using propensity score matching analysis. Am J Cancer Res 2018;8:1440-8. [PubMed]
- Goffredo P, Cheung K, Roman SA, et al. Can minimally invasive follicular thyroid cancer be approached as a benign lesion?: a population-level analysis of survival among 1,200 patients. Ann Surg Oncol 2013;20:767-72. [Crossref] [PubMed]
- Dralle H, Machens A. Surgical approaches in thyroid cancer and lymph-node metastases. Best Pract Res Clin Endocrinol Metab 2008;22:971-87. [Crossref] [PubMed]
- Nicolson NG, Murtha TD, Dong W, et al. Comprehensive Genetic Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent of Histology. J Clin Endocrinol Metab 2018;103:2640-50. [Crossref] [PubMed]
- Vogelstein B, Papadopoulos N, Velculescu VE, et al. Cancer genome landscapes. Science 2013;339:1546-58. [Crossref] [PubMed]
- O'Neill CJ, Vaughan L, Learoyd DL, et al. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur J Surg Oncol 2011;37:181-5. [Crossref] [PubMed]
- Sugino K, Ito K, Nagahama M, et al. Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid 2011;21:751-7. [Crossref] [PubMed]
- Kim HJ, Sung JY, Oh YL, et al. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head Neck 2014;36:1695-700. [Crossref] [PubMed]
- Podda M, Saba A, Porru F, et al. Follicular thyroid carcinoma: differences in clinical relevance between minimally invasive and widely invasive tumors. World J Surg Oncol 2015;13:193. [Crossref] [PubMed]
- Ríos A, Rodríguez JM, Ferri B, et al. Prognostic factors of follicular thyroid carcinoma. Endocrinol Nutr 2015;62:11-8. [Crossref] [PubMed]
- Su DH, Chang TC, Chang SH. Prognostic factors on outcomes of follicular thyroid cancer. J Formos Med Assoc 2019;118:1144-53. [Crossref] [PubMed]
- Duan H, Liu X, Ren X, et al. Mutation profiles of follicular thyroid tumors by targeted sequencing. Diagn Pathol 2019;14:39. [Crossref] [PubMed]
- Sugino K, Kameyama K, Ito K, et al. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid 2012;22:798-804. [Crossref] [PubMed]
- Ito Y, Hirokawa M, Masuoka H, et al. Prognostic factors of minimally invasive follicular thyroid carcinoma: extensive vascular invasion significantly affects patient prognosis. Endocr J 2013;60:637-42. [Crossref] [PubMed]
- Stenson G, Nilsson IL, Mu N, et al. Minimally invasive follicular thyroid carcinomas: prognostic factors. Endocrine 2016;53:505-11. [Crossref] [PubMed]
- Dai Y, Miao Y, Zhu Q, et al. Expression of long non-coding RNA H19 predicts distant metastasis in minimally invasive follicular thyroid carcinoma. Bioengineered 2019;10:383-9. [Crossref] [PubMed]
- Ito Y, Hirokawa M, Masuoka H, et al. Distant metastasis at diagnosis and large tumor size are significant prognostic factors of widely invasive follicular thyroid carcinoma. Endocr J 2013;60:829-33. [Crossref] [PubMed]
- Dionigi G, Kraimps JL, Schmid KW, et al. Minimally invasive follicular thyroid cancer (MIFTC)--a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 2014;399:165-84. [Crossref] [PubMed]
- Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81:1-122. [Crossref] [PubMed]
- Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clin Cancer Res 2013;19:2283-8. [Crossref] [PubMed]
- Musholt TJ, Staubitz JI, Antonio Cámara RJ, et al. Detection of RET rearrangements in papillary thyroid carcinoma using RT-PCR and FISH techniques - A molecular and clinical analysis. Eur J Surg Oncol 2019;45:1018-24. [Crossref] [PubMed]
- Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab 2003;88:2318-26. [Crossref] [PubMed]
- Nikiforova MN, Biddinger PW, Caudill CM, et al. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am J Surg Pathol 2002;26:1016-23. [Crossref] [PubMed]
- McHenry CR, Phitayakorn R. Follicular adenoma and carcinoma of the thyroid gland. Oncologist 2011;16:585-93. [Crossref] [PubMed]
- Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 2009;69:4885-93. [Crossref] [PubMed]
- Zhang Y, Yu J, Lee C, et al. Genomic binding and regulation of gene expression by the thyroid carcinoma-associated PAX8-PPARG fusion protein. Oncotarget 2015;6:40418-32. [Crossref] [PubMed]
- Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 2013;381:1058-69. [Crossref] [PubMed]
- Genutis LK, Tomsic J, Bundschuh RA, et al. Microsatellite Instability Occurs in a Subset of Follicular Thyroid Cancers. Thyroid 2019;29:523-9. [Crossref] [PubMed]
- Migdalska-Sęk M, Czarnecka KH, Kusiński M, et al. Clinicopathological Significance of Overall Frequency of Allelic Loss (OFAL) in Lesions Derived from Thyroid Follicular Cell. Mol Diagn Ther 2019;23:369-82. [Crossref] [PubMed]
Cite this article as: Staubitz JI, Musholt TJ. Current surgical management of follicular thyroid carcinoma. Ann Thyroid 2020;5:23.


