
Management of medullary thyroid carcinoma
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine tumor accounting for 3–10% of all thyroid cancers. It arises from the parafollicular C-cells and secretes calcitonin (CT), the most sensitive marker of MTC (1,2).
MTC mostly presents as sporadic between the fourth and sixth decades of life. Hereditary MTC, caused by RET germline mutations and transmitted as an autosomal dominant trait, occurs in 20–30% of cases, usually at young age. The hereditary variant may present as isolated familial MTC (FMTC, considered a variant of MEN 2A), or syndromic multiple endocrine neoplasm (MEN) type 2, characterized by MTC in combination with pheochromocytoma (PHEO) and primary hyperparathyroidism (MEN 2A) or in combination with PHEO, multiple mucosal neuromas and marfanoid habitus (MEN 2B) (3).
Strong genotype-phenotype associations regarding age at onset and tumor aggressiveness have been described for specific germline RET mutations.
Prognosis for MTC is worse than differentiated thyroid cancer due to early locoregional spread of disease and the lack of effective systemic therapy. Metastases of MTC occur early to both paratracheal and lateral cervical lymph nodes. Distant metastases may involve liver, lungs, bones and, less frequently, brain and skin (4).
The diagnosis of MTC, the staging of disease, and the exclusion of associated syndromic diseases are pivotal to plan the best therapeutic strategy.
The purpose of this review is to describe the management and treatment of patients with MTC, focusing on biochemical and imaging diagnostic tools, preoperative work-up, treatment and prophylactic surgery for hereditary variants.
Diagnosis and preoperative evaluation
Clinical and biochemical evaluation
The diagnosis of MTC follows the well-established diagnostic work-up of a thyroid nodule, including clinical, biochemical, morphological and pathological assessment.
During the clinical evaluation, a detailed medical history, and family history of endocrine diseases should be recorded (5). On physical examination, patients with MTC often present an asymptomatic solitary palpable thyroid mass, whose extent should be carefully determined as well as the presence of neck lymphadenopathy (6). Loco-regional symptoms such as dysphagia, coughing, hoarseness and dyspnea are usually associated with local advanced disease. In patients with metastatic MTC, diarrhea or flushing may represent one of the first clinical consequences of the excessive CT production. In very rare cases, Cushing’s syndrome, due to ectopic adrenocorticotropin hormone production of C-cells, has been described (7).
Moreover, clinical features of hereditary syndromic MTC, like arterial hypertension, the presence of multiple mucosal neuromas and intestinal ganglioneuromas (Hirschsprung’s disease), or marfanoid habitus should be evaluated especially in young patients (4). Clinical characteristics of catecholamines hypersecretion, such as paroxysmal arterial hypertension crisis, headache, episodes of copious sweating, palpitations, pallor and anxiety, should also be considered (3-5).
A complete thyroid hormonal assessment is required to assess and/or confirm the diagnosis of MTC. The most accurate biomarker of MTC is CT, a cleavage product peptide hormone of procalcitonin secreted in response to an increase in serum calcium from the parafollicular C-cells (8). Since neoplastic C-cells express and secrete CT, preoperative CT levels correlate significantly with the C-cells mass and therefore with MTC diameter at surgical pathology (9). Moreover, regular assessment of CT in subjects diagnosed with nodular goiter could lead to an early detection of MTC, this resulting in a positive prognostic impact in several series (10-12). However, although its cost-effectiveness has been clearly demonstrated, the systematic measurement of CT is done routinely in Europe but still not universally recommended by Scientific Societies. In fact, despite the high sensitivity in detecting MTC, its specificity is lowered by false positive results that may occur in several clinical conditions, such as male gender, children during the first 6 months of life, chronic renal failure, autoimmune thyroiditis, hypergastrinemia, sepsis, type 1A pseudohypoparathyroidism and macrocytosis (3,13). In these cases, however, provocative testing with potent secretagogues agents, such as intravenous calcium or pentagastrin, may disclose the presence of MTC. Nowadays, the most widely used stimulation method is calcium test, based on measurements of CT after acute administration of calcium gluconate. The extracellular calcium-sensing receptor (CaSR), expressed either in normal and neoplastic C-cells, senses the acute increase of calcium and stimulates acute CT secretion. This test provides reliable results and it is better tolerated than pentagastrin test (14-16). However, an accurate personal and pharmacological history, a thorough cardiovascular assessment and the measurement of serum electrolytes should be performed before the test and a continuous cardiac monitoring is recommended during the procedure to allow a prompt intervention in case of cardiac arrhythmia (3,17).
Finally, in some patients, a slight increase of serum CT may be attributed to other medical conditions than MTC, such as gastrointestinal neuroendocrine tumor or small or large cell lung cancers. In these cases, calcium or pentagastrin stimulation test does not evoke a significant peak of serum CT as in MTC.
Serum Procalcitonin, even if potentially affected by concomitant bacterial infection, may be useful in the management of MTC in certain clinical condition causing falsely elevated serum CT levels (18).
Carcinoembryonic antigen (CEA) is not a specific marker for MTC, and therefore is not sufficiently reliable for a timely diagnosis. However, CEA should be assessed with serum CT, since higher levels of CEA correlate with more advanced stage of MTC. Potential causes of falsely increased serum CEA are smoking tobacco, gastrointestinal tract inflammatory diseases or benign lung disease (3,4,19).
Other biochemical parameters that should be preoperatively collected are measurements of serum parathormone, calcium, phosphate, and 24 hours urine metanephrines and catecholamines, to exclude the presence of hereditary MTC and therefore the other associated diseases, in particular the presence of PHEO (3).
Diagnostic imaging and cytology
Ultrasound (US) is the preferred imaging modality for the evaluation of thyroid nodules and therefore it is the first line imaging technique for MTC diagnosis and staging. The majority of MTC shows the same US features of malignant nodules, including marked hypoechogenicity, indistinct margins, microcalcifications and increased Cvascularity. In patients with proven MTC, US evaluation with color and power Doppler techniques should also exclude the metastatic involvement of regional lymph nodes (20).
Cytology by fine-needle aspiration biopsy (FNAC) displays a poor accuracy in MTC detection, due to morphologic heterogeneity of MTC histological variants. As demonstrated by a meta-analysis of fifteen studies involving 641 cases of FNAC for MTC, the detection rate of MTC on cytology was only 56.4% (21). MTC cells may be epithelioid, plasmacytoid, polygonal, or spindle-shaped, usually with a weakly cohesive pattern. Moreover, eccentric nuclei with chromatin granularity, typical of neuroendocrine tumors, and amyloid deposits may be present (22). The diagnostic accuracy of FNAC may be increased by CT assay in the washout fluid of FNAC or by performing immunocytochemistry for CT, chromogranin or CEA (23). Measurement of CT in the wash-out fluid of FNAC of suspected cervical lymph nodes should be performed to confirm and stage the disease and to guide the extent of lymph node dissection.
Since the degree of CT elevation in MTC correlates well with distant metastatic spread, in presence of high CT levels and documented cervical nodal metastases additional imaging should be pursued in order to evaluate distant metastatic sites. In this context, contrast-enhanced computed tomography (CET) of the neck and chest is recommended to assess the extension of neck and mediastinal spread. Moreover, triphasic CET or contrast-enhanced magnetic resonance imaging (MRI) of the liver and MRI or bone scintigraphy for axial skeletal lesions should be done to rule out distant metastases frequently present in the liver and bone (3,19,24).
Based on the most recently revised American Thyroid Association (ATA) and on the National Comprehensive Cancer Network guidelines, 18-fluoro-deoxyglucose positron emission tomography (FDG-PET) and DOPA-PET scan are not recommended for initial work-up (3). However, in presence of very high levels of CT and CEA, DOPA-PET and FDG-PET are useful to determine the stage of disease and might show unknown metastatic disease (19) (Figures 1,2).
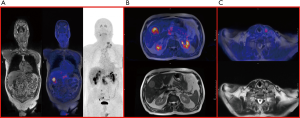
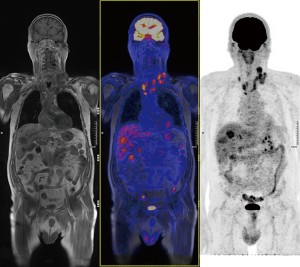
Genetics
RET protooncogene demonstrated to be the leading cause in the development of all hereditary and approximately 50% of sporadic MTC. RET is a 21-exons gene located on chromosome 10q11.2 and encodes a single-pass transmembrane receptor of the tyrosine kinase family (2,25). It is identified in cells derived from the branchial arches (parathyroid), the neural crest (brain, parasympathetic and sympathetic ganglia, thyroid C-cells, adrenal medulla, and enteric ganglia), and the urogenital system (26,27).
Hereditary MTC is caused by germline autosomal-dominant gain-of-function RET mutations, and for every known specific genotype a different phenotype has been identified. Mutations causing strong RET proto-oncogene activation are responsible for more severe clinic-pathological features of MTC than moderate overactivations. Based on that, the last ATA guidelines (3) reported three different risk categories (highest, high and moderate risk) depending on the genotype (Table 1).
Table 1
| MTC risk level | RET mutation | Exon | Incidence of (up to) | Timing of prophylactic thyroidectomy |
|
|---|---|---|---|---|---|
| PHEO | HPTH | ||||
| Moderate risk | G533C | 8 | ~10% | ~0% | >5 years of age if normal b + stCT |
| C609F/G/R/S/Y | 10 | ~30% | ~10% | ||
| C611F/G/S/Y/W | 10 | ~30% | ~10% | ||
| C618F/R/S | 10 | ~30% | ~10% | ||
| C620F/R/S | 10 | ~30% | ~10% | ||
| C630R/Y | 11 | ~30% | ~10% | ||
| D631Y | 11 | ~50% | ~0% | ||
| K666E | 11 | ~10% | ~0% | ||
| E768D | 13 | ~0% | ~0% | ||
| L790F | 13 | ~10% | ~0% | ||
| V804L | 14 | ~10% | ~10% | ||
| V804M | 14 | ~10% | ~10% | ||
| S891A | 15 | ~10% | ~10% | ||
| R912P | 16 | ~0% | ~0% | ||
| High risk | C634F/G/R/S/W/Y | 11 | ~50% | ~30% | ≤5 years of age |
| A883F | 15 | ~50% | ~0% | ||
| Highest risk | M918T | 16 | ~50% | ~0% | ASAP (≤1 year of age) |
MTC, medullary thyroid carcinoma; PHEO, pheochromocytoma; HPTH, primary hyperparathyroidism; b + stCT, basal and stimulated serum calcitonin; ASAP, as soon as possible.
The majority of patients with MEN 2B develop a de novo germline mutation in codon 918 of exon 16 and rarely in codon 883 of exon 15. Patients with MEN 2B develop MTC very early during infancy, usually before 5 years of age. MTC associated with MEN 2B is the most aggressive form of MTC, with an early spread to lymph nodes and distant metastases at diagnosis. Patients with MEN 2B also develop PHEO in about 50% of cases, and present a typical phenotype characterized by a marfanoid habitus, musculoskeletal manifestations and ganglioneuromatosis causing abdominal pain, constipation and megacolon (28).
MEN 2A/FMTC accounts for 80% of hereditary MTC. In 95% of patients MEN 2A mutations involve exon 10 (codons 609, 611, 618, 620) and exon 11 (codon 634). In this setting, MTC occurs in virtually all cases, PHEO in up to 50% and primary hyperparathyroidism in up to 30% of patients, depending on the RET codon mutation (Table 1). Other associated features include cutaneous lichen amyloidosis, and Hirschsprung’s disease (3).
RET genetic testing should be performed in all newly discovered MTCs since up to one third of patients with presumed sporadic MTC actually may have an inherited form. Moreover, in case of positive genetic result, all first-degree family members should be tested.
Genetic and/or biochemical screening is required prior to any intervention to exclude the coexistence of PHEO and primary hyperparathyroidism (3).
Somatic RET mutations have been also identified in sporadic MTC. Mutations in codon 918 occur in about 25–33% of sporadic MTC, and are associated with an aggressive clinical behavior and poor outcome. Other somatic RET mutations involving codons 618, 634, 768, 804 and 883 and partial deletion of RET gene have been rarely identified (29,30).
Pathology
At histology, MTC may have the characteristic morphology of neuroendocrine tumors, demonstrating the typical solid and nesting growth pattern. Amyloid deposition, derived from pre-procalcitonin deposits, is highly characteristic. However, pseudopapillary and oncocytic variants may mimic differentiated thyroid tumors, resembling follicular neoplasms or Hürthle cell carcinoma. Spindle-cell and giant-cell variants can be mistaken for sarcomas or anaplastic carcinomas, and rare but aggressive small-cell types resemble lymphomas.
C-cells hyperplasia should also be assessed since it may be correlated to germline RET mutation and inherited disease.
Histology should be confirmed by chromogranin, CT, and CEA immunostaining. Moreover, MTC molecular proliferative profile, including Ki-67 expression and somatic RET mutation analysis, is a useful tool for predicting patient survival independently from other classic prognostic factors (31).
Finally, the tumor should be appropriately staged using the American Joint Committee on Cancer (TNM Classification) system (32) (Table 2).
Table 2
| Stage | TNM stage |
|---|---|
| I | T1N0M0 |
| II | T2/T3 N0 M0 |
| III | T1/T2/T3 N1a M0 |
| IV | |
| A | T1/T2/T3 N1b M0; T4a AnyN M0 |
| B | T4b Any N M0 |
| C | Any T Any N M1 |
T1, tumor size ≤2 cm; T2, tumor size 2–4 cm; T3, tumor size >4 cm limited to the thyroid or any tumor with minimal extrathyroidal extension; T4a, tumor of any size with invasion of the adjacent soft tissue, trachea, larynx, esophagus or recurrent laryngeal nerve; T4b, tumor of any size with invasion of the prevertebral fascia or encasing carotid and mediastinal vessels; N1a, metastases to central neck lymph nodes; N1b, metastases to lateral neck or upper mediastinal lymph nodes; M1, distant metastases.
Surgical treatment
The adequacy of initial surgical treatment for MTC is of paramount prognostic importance. Total thyroidectomy and cervical lymph node dissection is considered the standard of treatment (Figure 3). Total thyroidectomy is advocated for both sporadic and hereditary MTC since multicentric and bilateral MTC is observed in 30% of sporadic and in nearly 100% of hereditary cases (3,4). A compartment-oriented lymph node dissection should be standard practice to achieve a curative treatment and disease control in the neck, at least in patients with node-positive MTC (33). Selective dissections of pathological enlarged lymph nodes have shown poor effective results in normalizing serum CT levels.
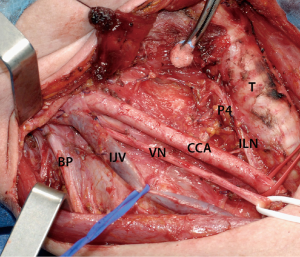
The current ATA guidelines (3) recommend, at initial surgery, a proper lymph node dissection of central neck compartment (level VI). A central neck node dissection should be performed in all cases, even in absence of clinical or imaging evidence of lymph node metastases, given the high risk of metastatic lymph node involvement at diagnosis and the high risk of neck recurrence. Moreover, an adequate clearance of the central neck compartment is crucial to avoid potential risks linked to reoperation. In fact, surgical morbidity related to thyroidectomy, including laryngeal nerve injuries and hypoparathyroidism, increases significantly during repeated neck operations in a scarred operative field (34).
Controversies exist in literature about the surgical management of lateral neck lymph node compartments. Lymph nodes of the ipsilateral neck compartments are involved in more than 60% of cases at diagnosis. The risk of lymph node metastases seems to be greater with the increase of diameter of the tumors (20–30% of cases with tumors <1 cm, 50% with a tumor 1–4 cm in diameter, and up to 90% with a tumor >4 cm or with a T4 tumor) (35,36). According to the current ATA guidelines (3) and the majority of authors, lateral neck lymph node dissection should be performed based mostly on presurgical US results, avoiding “prophylactic” dissection. However, some authors argue in favor of an elective lateral neck dissection according to serum CT levels. In fact, in a series of 300 consecutive patients operated for MTC, basal serum CT levels exceeding 20 pg/mL were gradually associated with lymph node metastases. Metastases were found in ipsilateral central and ipsilateral lateral neck in presence of serum CT >20 pg/mL, contralateral central neck (>50 pg/mL), contralateral lateral neck (>200 pg/mL) and in the upper mediastinum (>500 pg/mL) (37). Therefore, the ATA guidelines suggest the possibility of performing a preventive ipsilateral lateral lymph node dissection when basal CT levels are above 20 pg/mL, associated with a contralateral lateral neck dissection if preoperative imaging shows ipsilateral lateral neck involvement and basal CT is greater than 200 pg/mL.
In conclusion, the appropriate surgical management of lateral neck compartment in case of MTC remains debatable and should be discussed case by case in a multidisciplinary team of experts in the field including experienced endocrine surgeons. In fact, surgical morbidity associated with lateral neck lymph node dissection is not negligible, including lymphatic leakage (0.5–8%), and damage to the spinal accessory nerve with resulting shoulder dysfunction (25–50%) (38,39).
Surgery, when technically feasible, is also the first treatment in case of local advanced MTC. In this setting, however, the need of a more extensive surgery should be balanced against the increased risk of surgical morbidity. More aggressive surgical treatment with wide resection of involved neck structures may be appropriately performed in selected patients, according to age, survival rate, patient’s life expectancy, and coexistence of other medical conditions. However, function-preserving approaches to maintain speech, swallowing and parathyroid function are preferred, since a radical or debulking surgery may be unable to ensure a significant prognostic improvement (5). External radiotherapy should be considered when a radical surgery is unfeasible or in case of severe local extratumoral spreading (3).
In case of hereditary MTC, PHEO must be excluded prior to any surgical procedure, and in case of coexistence, PHEO should be treated first (3).
Persistent and recurrent disease
Only scant data and no randomized clinical trials about the prospects of cure for patients with persistent or recurrent MTC are available. In absence of distant metastases, surgery for persistent or recurrent loco-regional disease should be considered in patients with pathological lymph nodes at US associated with high CT levels in the washout fluid of FNAC and/or positive cytology (40). Before planning a reoperation, the potential chance of cure or disease control should be balanced against the potential surgical morbidity that is significantly higher during reoperation in a scarred tissue. Complications include permanent recurrent laryngeal nerve palsy and hypoparathyroidism, thoracic duct leak, injury to the phrenic nerve, brachial plexus and spinal accessory nerve. Therefore, a proper evaluation of the vocal cords with laryngoscopy before reoperation is needed.
In a study of Machens et al. (41), cure rate after a systematic lymph node dissection for persistent MTC seems to correlate with CT levels before reoperation and the number of metastatic lymph nodes removed at initial surgery. Patients with persistent/recurrent MTC with CT levels of 1,000 pg/mL or lower and 5 or less lymph nodes metastases removed at initial operation can achieve biochemical cure in the range of 18% to 44% with acceptable surgical morbidity.
In case of central neck recurrence, the space between the carotid and trachea can be more safely and easily reached through intact tissue planes by a lateral approach, during which strap muscles are mobilized laterally off the carotid. In general, limited dissections, such as resection of only grossly metastatic lymph nodes and ‘‘berry picking’’, should be avoided unless there was a prior compartment-oriented dissection.
Prophylactic thyroidectomy
DNA-based RET genetic screening of family members at risk of hereditary MTC is crucial to plan a timely surgical treatment. Prophylactic thyroidectomy, i.e., early removal of the thyroid gland in children who inherited a mutated RET allele, has become standard management worldwide and aims to provide a definitive cure before MTC develops, avoiding life-long oncological follow-up (3). However, in many cases small foci of MTC may be already present at the time of thyroidectomy.
Because of the close genotype-phenotype correlation, the timing of prophylactic thyroidectomy varies according to the different categories of RET mutations (Table 1).
Based on this classification, MEN 2B patients with the highest-risk mutation category in codon M918T should have a total thyroidectomy with central neck dissection possibly the first year of life. MEN2A patients in the high-risk category should be evaluated annually by physical examination, neck US and serum CT levels assessment starting at 3 years of age. In these patients, total thyroidectomy should be performed at or before 5 years of age. Children in the moderate-risk category develop MTC at a more variable age and prophylactic thyroidectomy can be postponed until later childhood or young adulthood, depending primarily on serum CT levels. Moreover, children with only slightly elevated CT levels can undergo total thyroidectomy without central neck lymph node dissection, reducing the risk of surgical morbidity (42,43). In fact, being preventive surgery, prophylactic thyroidectomy should keep surgical morbidity to a minimum. The parathyroid glands and the recurrent laryngeal nerves should be identified and preserved before dissecting the thyroid gland off its bed. Children and infants present small sized parathyroid glands that are sometimes not easy to identify and distinguish from the adjacent lymph fatty tissue. In fact, hypoparathyroidism is the most common surgical complication in these patients. To this purpose, optical magnification and autograft of devascularized parathyroid glands might be very useful. Moreover, surgical complications are significantly minimized when experienced surgeons perform the thyroidectomy (43). Hence, management of children with MEN2A or MEN2B should take place in tertiary care centers by a dedicated team of specialists.
Prognosis and long-term follow-up
Prognosis of MTC strictly depends on stage of disease at diagnosis. Patients at stage I and II at diagnosis have a significantly better survival and cure rate compared to those at stage III and IV (3,44). On univariate analysis, prognosis significantly depends on age at diagnosis, male sex, stage of disease, lymph nodes metastases and distant metastases. However, at multivariate analysis, the strongest prognostic factor still remains stage of disease at diagnosis (10,44). Even if prognosis of MTC is worse than of differentiated thyroid cancer, in recent decades a significant increase of survival and cure rate for MTC has been observed. This increase seems to be correlated to early detection of cases by wide screening of CT in thyroid nodules for sporadic MTC and RET genetic screening for hereditary MTC (11,45,46).
Postoperative follow-up includes periodic measurements of serum CT and CEA levels, starting at least 3 months after initial surgery to avoid a misleading interpretation of the results due to CT long half-life, which is even longer for CEA (47). Patients biochemically cured, whose serum CT is undetectable and CEA is normal, can be evaluated with a 6 months follow-up for the first year and annually thereafter, with an excellent prognosis with a disease recurrence rate over 5–10 years ranging from <1 to 8.5%, and a 5-year survival rate of 97–99% (48,49). Patients not biochemically cured, who present high postoperative serum CT levels, should be monitored with neck US and CT measurements every 6 months, to estimate the CT doubling time. CT doubling time has been shown to be a significant predictor of survival in patients with MTC. In a retrospective study of Barbet et al. involving 65 MTC patients, the 5- and 10-year survival rates in patients with CT doubling times less than 6 months were significantly lower compared to those with doubling times between 6 and 24 months (50).
Patients with CT levels higher than 150 pg/mL should be evaluated for distant metastatic disease with CET of the chest, contrast-enhanced MRI or CET of the liver, and bone scintigraphy or MRI of the pelvis and axial skeleton. Functional imaging techniques such as DOPA-PET or FDG-PET seem to be superior to conventional imaging in detecting MTC metastases. DOPA-PET has a higher sensitivity compared to FDG-PET in evaluating the extent of disease, but FDG-PET positive findings correlate better with disease progression and with a lower survival (51).
Metastatic MTC and other treatments
Distant metastases of MTC are present at diagnosis in 4–17% of patients, and will be detected during follow-up in another 18–38% of cases (19,52).
Distant metastases of MTC are usually found in the liver, lungs, bones and, less frequently, the brain and soft tissues (19). Subjects affected by metastatic MTC usually have poor outcome, with 26% of survival at 5 years and 10% at 10 years (53). However, distant metastases can be sometimes found at an early stage when small and may remain stable over years or decades. Focal treatment approaches of metastatic MTC should be considered in case of local neck advanced MTC or oligometastatic disease. Surgery could be indicated for bone metastases causing orthopedic or neurological complications, and in technically operable metastases of the brain, lungs or liver. Stereotactic radiosurgery for brain metastases and chemoembolization for liver metastases may be considered. Radiofrequency ablation of liver metastases can be performed for local tumor control or in case of severe uncontrolled diarrhea (3,19).
MTC metastases are often multiple in each involved organ and, therefore, may require other adjuvant and palliative therapeutic options. External radiotherapy seems to be effective for multiple brain metastases and in reducing rapidly pain for bone metastases that are not amenable to surgery.
Several trials on the effect of somatostatin analogues on MTC have been published, but somatostatin analogues with a high affinity for SSTR2 and SSTR5 (octreotide and lanreotide) do not seem to improve survival and in some cases cause complications such as flushing and diarrhea (54,55).
Systemic chemotherapy usually provides partial or transient responses. Doxorubicin, used either alone or in combination with cisplatinum, is the most used cytotoxic drug in MTC patients, with a response rate of 0–20% (56). The combination of 5-fluorouracil with dacarbazine seemed to be more effective with a response rate of about 20% (57).
Other combinations with 5-fluorouracil, dacarbazine, streptozocin, cyclophosphamide and vincristine have been described; however, none of these compounds is commonly used.
Nowadays, standard chemotherapeutic agents are not considered first-line therapy for patients with persistent or recurrent MTC given their low efficacy and the development of more targeted molecular compounds (3).
Targeted therapies
A greater understanding of the molecular oncogenesis of MTC has led to the identification of novel molecular targets for treatment of locally advanced and/or metastatic disease. The two main targets of the drugs used in patients with MTC, the tyrosine kinase inhibitors (TKIs), are the kinases of RET and vascular endothelial growth factor receptor-2 (VEGFR2).
The TKIs work by blocking the tyrosine kinase receptor, preventing its autophosphorylation and activation of several signaling pathways.
Several TKIs have demonstrated high response rates in phase II trials in patients with metastatic MTC. Two compounds, vandetanib (58) and cabozantinib (59,60), have been investigated in large prospective, randomized, double-blind phase III clinical trials, that included patients with locally advanced or metastatic MTC that was progressive or aggressive and symptomatic. All these drugs have substantial toxic effects, such as diarrhea, fatigue, hypertension and prolongation of the QTc interval. The choice of the drug to be used as first-line treatment (vandetanib or cabozantinib) is still debatable, since both are active and both have several toxic effects.
TKIs should be considered in patients with progressive metastatic disease and as first-line systemic therapy in patients with advanced progressive MTC. Both vandetanib and cabozantinib demonstrated significant effects on tumor progression compared with placebo and they were approved for use from the US Food and Drug Administration and European Medicines Agency. They should be avoided in patients with biochemical disease but absent imaging evidence of disease, and in those with no evidence of progression on imaging.
Unfortunately, clinical responses for advanced MTC are partial and patients may develop resistance to the drug and disease progression over time. Progression of disease might be associated with insufficient drug doses or with intra-tumor mechanisms; indeed, a RET mutation at codon 804 in the tumor cells might induce resistance to vandetanib (61).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for series “Recent Challenges in the Management of Thyroid Tumors”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-41). The series “Recent Challenges in the Management of Thyroid Tumors” was commissioned by the editorial office without any funding or sponsorship. MI served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol 1966;19:114-8. [Crossref] [PubMed]
- Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2a and FMTC. Hum Mol Genet 1993;2:851-6. [Crossref] [PubMed]
- Wells SA, Asa SL, Dralle H, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
- Pacini F, Castagna MG, Cipri C, et al. Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 2010;22:475-85. [Crossref] [PubMed]
- Konstantinidis A, Stang M, Roman SA, et al. Surgical management of medullary thyroid carcinoma. Updates Surg 2017;69:151-60. [Crossref] [PubMed]
- Moley JF. Medullary thyroid carcinoma: Management of lymph node metastases. J Natl Compr Canc Netw 2010;8:549-56. [Crossref] [PubMed]
- Choi HS, Kim MJ, Moon CH, et al. Medullary thyroid carcinoma with ectopic adrenocorticotropic hormone syndrome. Endocrinol Metab (Seoul) 2014;29:96-100. [Crossref] [PubMed]
- Costante G, Durante C, Francis Z, et al. Determination of calcitonin levels in C-cell disease: Clinical interest and potential pitfalls. Nat Clin Pract Endocrinol Metab 2009;5:35-44. [Crossref] [PubMed]
- Leboulleux S, Baudin E, Travagli JP, et al. Medullary thyroid carcinoma. Clin Endocrinol (Oxf) 2004;61:299-310. [Crossref] [PubMed]
- Torresan F, Cavedon E, Mian C, et al. Long-Term Outcome After Surgery for Medullary Thyroid Carcinoma: A Single-Center Experience. World J Surg 2018;42:367-75. [Crossref] [PubMed]
- Torresan F, Mian C, Cavedon E, et al. Cure and survival of sporadic medullary thyroid carcinoma following systematic preoperative calcitonin screening. Langenbecks Arch Surg 2019;404:411-9. [Crossref] [PubMed]
- Iacobone M, Niccoli-Sire P, Sebag F, et al. Can sporadic medullary thyroid carcinoma be biochemically predicted? Prospective analysis of 66 operated patients with elevated serum calcitonin levels. World J Surg 2002;26:886-90. [Crossref] [PubMed]
- Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol 2012;8:466-75. [Crossref] [PubMed]
- Doyle P, Düren C, Nerlich K, et al. Potency and tolerance of calcitonin stimulation with high-dose calcium versus pentagastrin in normal adults. J Clin Endocrinol Metab 2009;94:2970-4. [Crossref] [PubMed]
- Colombo C, Verga U, Mian C, et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow-up of medullary thyroid cancer. J Clin Endocrinol Metab 2012;97:905-13. [Crossref] [PubMed]
- Mian C, Perrino M, Colombo C, et al. Refining calcium test for the diagnosis of medullary thyroid cancer: Cutoffs, procedures, and safety. J Clin Endocrinol Metab 2014;99:1656-64. [Crossref] [PubMed]
- Fugazzola L. Baseline and stimulated calcitonin: Thresholds for the diagnosis of medullary thyroid cancer. Ann Endocrinol (Paris) 2019;80:191-2. [Crossref] [PubMed]
- Kratzsch J, Petzold A, Raue F, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem 2011;57:467-74. [Crossref] [PubMed]
- Hadoux J, Pacini F, Tuttle RM, et al. Management of advanced medullary thyroid cancer. Lancet Diabetes Endocrinol 2016;4:64-71. [Crossref] [PubMed]
- Lee S, Shin JH, Han BK, et al. Medullary thyroid carcinoma: Comparison with papillary thyroid carcinoma and application of current sonographic criteria. AJR Am J Roentgenol 2010;194:1090-4. [Crossref] [PubMed]
- Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: A meta-analysis. Clin Endocrinol (Oxf) 2015;82:280-5. [Crossref] [PubMed]
- Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol 2000;22:351-8. [Crossref] [PubMed]
- Trimboli P, Cremonini N, Ceriani L, et al. Calcitonin measurement in aspiration needle washout fluids has higher sensitivity than cytology in detecting medullary thyroid cancer: A retrospective multicentre study. Clin Endocrinol (Oxf) 2014;80:135-40. [Crossref] [PubMed]
- Giraudet AL, Vanel D, Leboulleux S, et al. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J Clin Endocrinol Metab 2007;92:4185-90. [Crossref] [PubMed]
- Mulligan LM, Kwok JBJ, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 1993;363:458-60. [Crossref] [PubMed]
- Nakamura T, Ishizaka Y, Nagao M, et al. Expression of the ret proto-oncogene product in human normal and neoplastic tissues of neural crest origin. J Pathol 1994;172:255-60. [Crossref] [PubMed]
- Iacobone M, Citton M, Viel G, et al. Surgical approaches in hereditary endocrine tumors. Updates Surg 2017;69:181-91. [Crossref] [PubMed]
- Castinetti F, Moley J, Mulligan L, et al. A comprehensive review on MEN2B. Endocr Relat Cancer 2018;25:T29-39. [Crossref] [PubMed]
- Romei C, Elisei R, Pinchera A, et al. Somatic mutations of the ret protooncogene in sporadic medullary thyroid carcinoma are not restricted to exon 16 and are associated with tumor recurrence. J Clin Endocrinol Metab 1996;81:1619-22. [PubMed]
- Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682-7. [Crossref] [PubMed]
- Mian C, Pennelli G, Barollo S, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: A useful tool for patient risk stratification. Eur J Endocrinol 2011;164:971-6. [Crossref] [PubMed]
- Amin M.B., Edge S.B., Greene F.L. et al., editor. AJCC Cancer Staging Manual. 8th Ed. New York: Springer, 2017.
- Tamagnini P, Iacobone M, Sebag F, et al. Lymph node involvement in macroscopic medullary thyroid carcinoma. Br J Surg 2005;92:449-53. [Crossref] [PubMed]
- Machens A, Dralle H. Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg 2013;257:751-7. [Crossref] [PubMed]
- Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma: Recommendations for extent of node dissection. Ann Surg 1999;229:880-7. [Crossref] [PubMed]
- Scollo C, Baudin E, Travagli JP, et al. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2003;88:2070-5. [Crossref] [PubMed]
- Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2655-63. [Crossref] [PubMed]
- Lorenz K, Abuazab M, Sekulla C, et al. Management of lymph fistulas in thyroid surgery. Langenbecks Arch Surg 2010;395:911-7. [Crossref] [PubMed]
- Sobol S, Jensen C, Sawyer W, et al. Objective comparison of physical dysfunction after neck dissection. Am J Surg 1985;150:503-9. [Crossref] [PubMed]
- Kebebew E, Kikuchi S, Duh QY, et al. Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg 2000;135:895-901. [Crossref] [PubMed]
- Machens A, Lorenz K, Dralle H. Time to calcitonin normalization after surgery for node-negative and node-positive medullary thyroid cancer. Br J Surg 2019;106:412-8. [PubMed]
- Pelizzo MR, Torresan F, Boschin IM, et al. Early, prophylactic thyroidectomy in hereditary medullary thyroid carcinoma: A 26-year monoinstitutional experience. Am J Clin Oncol 2015;38:508-13. [Crossref] [PubMed]
- Machens A, Elwerr M, Lorenz K, et al. Long-term outcome of prophylactic thyroidectomy in children carrying RET germline mutations. Br J Surg 2018;105:e150-7. [Crossref] [PubMed]
- Modigliani E, Cohen R, Campos JM, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: Results in 899 patients. Clin Endocrinol (Oxf) 1998;48:265-73. [Crossref] [PubMed]
- Machens A, Dralle H. Surgical cure rates of sporadic medullary thyroid cancer in the era of calcitonin screening. Eur J Endocrinol 2016;175:219-28. [Crossref] [PubMed]
- Mirallié E, Iacobone M, Sebag F, et al. Results of surgical treatment of sporadic medullary thyroid carcinoma following routine measurement of serum calcitonin. Eur J Surg Oncol 2004;30:790-5. [Crossref] [PubMed]
- Fugazzola L, Pinchera A, Luchetti F, et al. Disappearance rate of serum calcitonin after total thyroidectomy for medullary thyroid carcinoma. Int J Biol Markers 1994;9:21-4. [Crossref] [PubMed]
- Tuttle RM, Ganly I. Risk stratification in medullary thyroid cancer: Moving beyond static anatomic staging. Oral Oncol 2013;49:695-701. [Crossref] [PubMed]
- Lindsey SC, Ganly I, Palmer F, et al. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid 2015;25:242-9. [Crossref] [PubMed]
- Barbet J, Campion L, Kraeber-Bodéré F, et al. Prognostic impact of serum calcitonin and carcinoembryonic antigen doubling-times in patients with medullary thyroid carcinoma. J Clin Endocrinol Metab 2005;90:6077-84. [Crossref] [PubMed]
- Verbeek HHG, Plukker JTM, Koopmans KP, et al. Clinical relevance of18F-FDG PET and18F-DOPA PET in recurrent medullary thyroid carcinoma. J Nucl Med 2012;53:1863-71. [Crossref] [PubMed]
- Kebebew E, Ituarte PHG, Siperstein AE, et al. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139-48. [Crossref] [PubMed]
- Pelizzo MR, Boschin IM, Bernante P, et al. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 2007;33:493-7. [Crossref] [PubMed]
- Iten F, Müller B, Schindler C, et al. Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: A phase II clinical trial. Clin Cancer Res 2007;13:6696-702. [Crossref] [PubMed]
- Kraeber-Bodéré F, Goldenberg DM, Chatal JF, et al. Pretargeted radioimmunotherapy in the treatment of metastatic medullary thyroid cancer. Curr Oncol 2009;16:3-8. [PubMed]
- Shimaoka K, Schoenfeld DA, Dewys WD, et al. A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 1985;56:2155-60. [Crossref] [PubMed]
- Schlumberger M, Abdelmoumene N, Delisle MJ, et al. Treatment of advanced medullary thyroid cancer with an alternating combination of 5 FU-streptozocin and 5 FU-dacarbazine. The Groupe d’Etude des Tumeurs a Calcitonine (GETC). Br J Cancer 1995;71:363-5. [Crossref] [PubMed]
- Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639-46. [Crossref] [PubMed]
- Bertazza L, Sensi F, Cavedon E, et al. EF24 (a Curcumin Analog) and ZSTK474 Emphasize the Effect of Cabozantinib in Medullary Thyroid Cancer. Endocrinology 2018;159:2348-60. [Crossref] [PubMed]
- Carlomagno F, Guida T, Anaganti S, et al. Disease associated mutations at valine 804 in the RET receptor tyrosine kinase confer resistance to selective kinase inhibitors. Oncogene 2004;23:6056-63. [Crossref] [PubMed]
Cite this article as: Torresan F, Armellin C, Iacobone M. Management of medullary thyroid carcinoma. Ann Thyroid 2020;5:16.


