
The evolution and progress of the application of intraoperative monitoring in endoscopic thyroid surgery
Introduction on nerve monitoring
Intraoperative nerve monitoring (IONM) is an important adjunct in thyroid surgery in detecting recurrent laryngeal nerve (RLN) injury. The reported incidence of RLN injury in thyroid surgery varies with RLN paresis ranging from 2.6% to 5.9% and RLN paralysis ranging from 0.5% to 2.4%, making it one of the most common complications after surgery with medico-legal consequences (1,2). IONM started in the 1930s with the concept of direct visualization and dissection of the RLN during surgery and evolved into analysis of amplitude and latencies in the monitoring of evoked potentials using electromyography (EMG). IONM was standardized in 2011 with published guidelines from the International Neural Monitoring Group in order to ensure reproducible and verifiable results (3). IONM has evolved from application in open thyroid surgery to endoscopic thyroid surgery. This review focuses on the role of IONM in transoral endoscopic thyroidectomy vestibular approach (TOETVA) as it evolved from the open surgery approach.
Various technologies applied
The advent of IONM started with functional nerve verification (FNV) in the 1930s. Technologies that were applied began with direct visualization and older methods that relied on pressure changes on an inflated endotracheal tube when the nerve was stimulated (4). Methods of electrical stimulation of the nerve came into play in the 1980s, where they were based on observation or palpation of contraction of the effector muscles after stimulating the motor nerve with electricity (5). In 1985, James et al proposed a method of palpation with stimulation (6). More recently, electromyography (EMG) methods came into use. These methods can be divided in two groups: those that use needle electrodes directly inserted in the effector muscle and those that register potentials with surface electrodes located over the effector muscle. Barwell et al. introduced the use of a nerve integrity monitor (NIM) device (7). The most recent modification is continuous vagal stimulation, which puts the electrodes on the vagus nerve. The expected advantages of this method known as continuous IONM (CIONM) include continuous recording of the nerve signal that allows more specific control of the manipulation during all the steps of the surgery, indicating when the nerve is mobilized or touched, to avoid dangerous maneuvers (8). The idea behind such an approach stems from the fact that most nerve injuries are due to stretch injuries that can be modified in real time if adequate feedback is provided. The most feasible technology that is utilized today is the surface electrodes over the endotracheal tube, located over the effector muscles. While there are new technologies being developed, including the adaptation of CIONM, each of these technologies remains an adjunct to direct visualization of the RLN.
Variations in settings and outputs
The International Neural Monitoring Group (INMG) published a set of guidelines on the set up and established protocols for neural monitoring in 2011 in order to improve the quality of neural monitoring and to reduce inappropriate variations in monitoring technique (3). The goal of the published guidelines was to establish the setting and output for IONM so that results could be interpreted consistently and intraoperative electrophysiologic data could be correlated with postoperative glottic function. IONM was determined to be important in neural mapping, aiding in dissection of the nerve and for prognostication of post-operative neural function and the site of damage along the nerve if any (3).
RLN monitor settings were established according to the guidelines (3). Impedance values should be checked preoperatively. They should be less than 5 kΩ for each electrode with an imbalance of less than 1 kΩ between electrodes. Low impedance means good endotracheal tube contact with the effector muscles. High impedance should be corrected. The appropriate event threshold at 100 µV and a stimulator probe should be set on a value of 1 to 2 mA. Initial wave forms >100 micro volts establish an intact circuit. During dissection a loss of signal (LOS) is considered <100 micro volts that is not retrieved within 20 minutes. The positive predictive value for postoperative vocal fold weakness is 100% if this is the case. Increasing the amplitude >2 mA may be necessary in order to evoke stimulation of branches of the RLN, without due risk of injury from electrical stimulation. While these standards are established to create a uniformity in monitoring, the gold standard remains direct visualization of the nerve. Use of IONM requires pre and post-operative laryngoscopy in order to detect true incidence of vocal fold weakness.
Current application
The current gold standard remains direct visualization of the RLN. A meta-analysis done by Yang et al. demonstrated that for high risk (re-operative) thyroid surgeries and for those thyroidectomies done for malignancy, IONM was better than visualization alone in preventing temporary vocal fold paralysis (9). For permanent vocal fold paralyses this was not found to be significant. Furthermore, the meta-analysis was done on the majority of studies that are published including cohort, case studies and retrospective reviews. There is a dearth of strong randomized control studies that have been published on this subject. The variability in reporting and the non-standardization of protocols contribute to this. Dralle et al. has studied issues of statistical power necessary to prove that rates of paralysis are lower with the application of neural monitoring. His studies have suggested that a researcher would need 9 million patients per arm for benign multinodular goiter and approximately 40,000 patients per arm for thyroid cancer for such studies to be conducted with statistical power if typical rates of nerve paralysis are used for calculation (10).
One of the largest surveys undertaken demonstrate the rate of IONM usage in the United States between general surgeons and otolaryngologists alike is 40–45% (11). It is almost 95% in Germany, making it a routine tool used by thyroid surgeons in that country (12). Fellowship trained surgeons who utilized IONM are more likely to use it in practice, and this trend is increasing towards younger surgeons advocating for its use more routinely than prior (13). Overall, while the trends are changing, the published research still suggests that the gold standard is direct visualization. How this is being influenced in endoscopic thyroid surgery remains to be discussed in the next section.
Minimally invasive/remote access to the thyroid
Remote access is becoming common at some high-volume endocrine surgery centers. The emphasis on cosmesis and tailored patient satisfaction has spurred efforts to refine and adopt approaches to thyroid surgery which avoid a scar in the neck (14). Endoscopic thyroid surgery, in its multiple approaches, is gaining widespread acceptance worldwide after improvements and modifications in technique and route of access (15-18).
Miccoli was the first to propose a minimally invasive approach to the thyroid using endoscopes. multiple approaches have been described (15,19-24), but the TOETVA (17,18,25), the newest of the approaches, has several advantages—no scar in the neck, extensive inclusion criteria, unfettered access to the entire thyroid gland, laryngeal nerves, parathyroid glands and bilateral central neck, reproducible technique and a reliable postoperative course (20). This approach offers the potential of gaining an increased share of thyroidectomies performed.
Injury to the recurrent laryngeal and the superior laryngeal nerve can occur with the endoscopic thyroidectomy as with transcervical thyroidectomy (17,18,21,22). The approach to the laryngeal nerves is different with transcervical and the transoral endoscopic approaches. In transcervical thyroidectomy the superior laryngeal nerve is visualized along a trajectory parallel to the entry point of the nerve. Visual identification of the RLN remains the standard of care. Three approaches to the RLN are: lateral approach where the nerve is identified at the midpolar level (most commonly used), inferior approach—RLN identified at the thoracic inlet and superior approach—RLN identified at the ligament of Berry (least common) (23).
While performing the TOETVA the superior laryngeal nerve is not routinely visualized for two specific reasons: first, its superior and deep orientation are highly protected by the angle of dissection, and second, the high magnification and meticulous dissection obtained via the approach ensures that these branches are protected should any be in the course of the dissection. The RLN is identified primarily through the superior approach, near the nerve insertion. The disadvantage of this approach is the technical ability that is necessary to simultaneously retract the gland while using a laparoscopic instrument to dissect the nerve. Advantages include, once dissection of the nerve insertion has been completed, the relatively oblique course of the RLN reduces subsequent risk of injury. Perhaps even more important is the fact that the retrograde dissection, in-plane with the nerve reduces traction and may theoretically result in reduced injury rates. Due to the excellent safety profile of the standard approach, it will take a high surgical volume to demonstrate equivalency.
IONM technique in endoscopic thyroid surgery
Techniques for neuromonitoring are validated for use in open thyroid surgery—intermittent and continuous. Intermittent intraoperative monitoring of the RLN is the commonly employed technique in most centers (25). However, there are no accepted standards for IONM use in endoscopic/robotic thyroid surgery.
The probes utilized in TOETVA have been modified from open thoracic surgery, where the long thoracic probe is available. Certain centers have demonstrated the feasibility of percutaneous puncture of the skin over the operative area and passing the probe into the operative field for nerve monitoring (26-28). The efficacy and verification of using dissecting instruments and cautery hooks is not adequately studied, but adaptations of these various tools has also been advocated by some authors (29,30). Another design is the flexible wire probes that can be introduced into the operative field using an endograsper (31). At the authors’ institution, a 23-cm ball tip long monopolar stimulation probe (Medtronic) is introduced through the side port. It is versatile, has a malleable tip, is readily available at our institution, and is atraumatic (32). Anecdotally, we have noted that the wave form amplitudes vary from the standard, open, approach, perhaps because the ball tip probe dispersed the delivery of stimulus compared to the fine point traditionally used by our group in open surgery. Probes for both intermittent IONM and CIONM have been used in TOETVA (see Figure 1). As with the intermittent technique, CIONM steps have been standardized for open surgery (34). Further investigation is necessary to better understand this information.
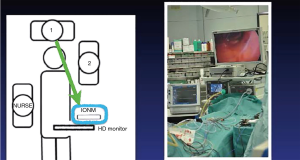
Current status of IONM in endoscopic thyroid surgery
IONM is fundamental to safe TOETVA. Like any new technology or technique, it is held to the benchmarks for safety like the gold standard, in this case, open thyroid surgery. Functional post-operative status of the RLN is a key indicator of safety. Hence, ensuring RLN integrity throughout endoscopic surgery is essential for the acceptance of this new technique.
IONM was first introduced in video-assisted thyroidectomy (VAT) in 2009 as a complementary technology to visual identification (35). In a subsequent study by the same author, data on 201 nerves at risk was examined with the application of IONM in VAT. The incidence of temporary vocal fold dysfunction was 6.5% and permanent RLN paralysis was 0.5%. This study elucidated the mechanisms of injury in VAT and compared it to mechanisms of injury in open surgery (36). Traction injury appeared to be the most common mode of injury at the region of ligament of Berry. Thermal injury follows traction injures due to increased use of energy devices. Injury to the RLN due to clamping and transection which can occur in open surgery is less common.
A recent review of neuromonitoring in endoscopic and robotic thyroidectomy looked at 522 nerves at risk. Just nine studies used IONM. The reports included ranged from the year 2009 to 2016. They found that adherence to the International Monitoring Study Group was low with only 30% of studies performing vagus nerve stimulation (3). Intermittent IONM was the technology used in more than 90% of the studies.
In a more recent study with transoral thyroidectomy 289 nerves at risk were analyzed with a temporary RLN paralysis rate of 7% with no permanent RLN palsy (18). Three new reports using continuous and intermittent IONM have been published over the last 24 months. In the North American and Italian series which included 27 patients looking at 34 nerves at risk, the North American group reported temporary palsy rate of 7%. They used intermittent IONM using a ball tip stimulator (17,37).
Literature on the monitoring of external branch of superior laryngeal nerve is sparse (38).
The advantages of the intermittent technique of IONM are: (I) helps in locating and mapping of the nerve; (II) aids in early recognition of injury and avoidance of further dissection in the area; (III) confirms that the nerve is functionally intact after dissection is complete; (IV) IONM could effectively localize the RLN more quickly, decrease the time to complete a lobectomy which could help in reducing costs related to the duration of surgery (26). Lee reported a quicker recovery of voice in patients where IONM was used in robotic thyroidectomy indicating IONM may have prevented further injury by alerting the surgeon of possible injury to the nerve after dissection (29).
The disadvantages of using IONM in endoscopic thyroid surgery may include user error and failure in identifying nerve tissue, ineffective stimulation with interference from electrocautery and injury to the distal RLN which may not be as fully mapped during endoscopic versus open surgery. The RLN in TOETVA is identified at the Ligament of Berry; the nerve is not fully mapped as in open surgery and therefore the intermittent monitoring of the RLN may not completely utilize the benefits of nerve monitoring.
A recent study has demonstrated the use of CIONM in TOETVA examining 28 nerves at risk (39). There were no nerve injuries reported. The advantages of real time monitoring of the functional status of the RLN during dissection may help endoscopic thyroid surgeons maneuver around the nerve, and suspend any moves that may propagate damage. This is especially relevant given the fact that surgeons are identifying the nerve at a less common site. The disadvantages of CIONM in TOETVA is that the vagus nerve is not routinely in the operative field. This may contribute to an increase in the operative time, and failure to map and identify the RLN. Intermittent monitoring may still need to be used thereby increasing the cost (39). Though the safety profile of CIONM has been demonstrated (39,40), it has not been widely adopted in open/endoscopic/robotic thyroidectomies probably due to the difficulty and reluctance of surgeons to undertake vagus nerve dissection (25).
IONM has helped us understand the mechanisms of injury during open and endoscopic surgery. Tactile sensation is blunted in endoscopic surgery which represents a disadvantage to most thyroid surgeons who are well trained in open thyroidectomy. Energy devices are an irreplaceable tool in TOETVA—they help in meticulous hemostasis and aid in dissection, vastly aiding the progress of the procedure. IONM may therefore serve us well in the recognition of RLN injury due to factors that are not obvious—such as excessive traction and thermal spread.
Just like in open thyroid surgery, IONM systems are not the gold standard yet but are complementary to visual identification of the nerve when used with video endoscopes (35).
The cost of IONM is a subject that has been identified in the course of its evolution and its routine use in low risk cases has been questioned (41). IONM use has a net benefit when it used in a total thyroidectomy as reported in a recent study based on a hypothetical patient (42). However, there are no studies at present that perform a comprehensive cost benefit analysis of IONM in endoscopic thyroid surgery (42).
Conclusions
IONM is an important part of thyroid surgery for most high-volume thyroid surgeons. It has been adapted and utilized in endoscopic thyroid surgery, as an adjunct that may be even more critical as the anatomy is not as familiar to neophyte endoscopic thyroid surgeons. The feasibility and adaptation of IONM in its intermittent or continuous forms has allowed surgeons to brave the advancement in minimally invasive thyroid surgery with a mind towards patient safety and strong outcomes against vocal fold weakness. While IONM is not the standard of care, it is widely used and is especially helpful with remote access thyroid approaches such as the TOETVA.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński, Hui Sun and Xiaoli Liu) for the series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.11.01). The series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. Dr. Tufano reports personal fees and other from Medtronic, personal fees from Hemostatix, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shindo M, Chheda NN. Incidence of vocal cord paralysis with and without recurrent laryngeal nerve monitoring during thyroidectomy. Arch Otolaryngol Head Neck Surg 2007;133:481-5. [Crossref] [PubMed]
- Kandil E, Noureldine SI, Abbas A, et al. The impact of surgical volume on patient outcomes following thyroid surgery. Surgery 2013;154:1346-52; discussion 1352-3. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Hvidegaard T, Vase P, Dalsgaard SC, et al. Endolaryngeal devices for perioperative identification and functional testing of the recurrent nerve. Otolaryngol Head Neck Surg 1984;92:292-4. [Crossref] [PubMed]
- Hillermann CL, Tarpey J, Phillips DE. Laryngeal nerve identification during thyroid surgery -- feasibility of a novel approach. Can J Anaesth 2003;50:189-92. [Crossref] [PubMed]
- James AG, Crocker S, Woltering E, et al. A simple method for identifying and testing the recurrent laryngeal nerve. Surg Gynecol Obstet 1985;161:185-6. [PubMed]
- Barwell J, Lytle J, Page R, et al. The NIM‐2 nerve integrity monitor in thyroid and parathyroid surgery. Br J Surg 1997;84:854. [Crossref] [PubMed]
- Ulmer C, Friedrich C, Kohler A, et al. Impact of continuous intraoperative neuromonitoring on autonomic nervous system during thyroid surgery. Head Neck 2011;33:976-84. [Crossref] [PubMed]
- Yang S, Zhou L, Lu Z, et al. Systematic review with meta-analysis of intraoperative neuromonitoring during thyroidectomy. Int J Surg 2017;39:104-13. [Crossref] [PubMed]
- Dralle H, Sekulla C, Haerting J, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 2004;136:1310-22. [Crossref] [PubMed]
- Horne SK, Gal TJ, Brennan JA. Prevalence and patterns of intraoperative nerve monitoring for thyroidectomy. Otolaryngol Head Neck Surg 2007;136:952-6. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 2012;99:1089-95. [Crossref] [PubMed]
- Marti JL, Holm T, Randolph G. Universal use of intraoperative nerve monitoring by recently fellowship-trained thyroid surgeons is common, associated with higher surgical volume, and impacts intraoperative decision-making. World J Surg 2016;40:337-43. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Wound morbidity in mini-invasive thyroidectomy. Surg Endosc 2011;25:62-7. [Crossref] [PubMed]
- Richmon JD, Holsinger FC, Kandil E, et al. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg 2011;5:279-82. [Crossref] [PubMed]
- Park JO, Sun DI. Transoral endoscopic thyroidectomy: our initial experience using a new endoscopic technique. Surg Endosc 2017;31:5436-43. [Crossref] [PubMed]
- Russell JO, Clark J, Noureldine SI, et al. Transoral thyroidectomy and parathyroidectomy–A North American series of robotic and endoscopic transoral approaches to the central neck. Oral Oncol 2017;71:75-80. [Crossref] [PubMed]
- Anuwong A, Sasanakietkul T, Jitpratoom P, et al. Transoral endoscopic thyroidectomy vestibular approach (TOETVA): indications, techniques and results. Surg Endosc 2018;32:456-65. [Crossref] [PubMed]
- Miccoli P, Materazzi G. Minimally invasive, video-assisted thyroidectomy (MIVAT). Surg Clin North Am 2004;84:735-41. [Crossref] [PubMed]
- Dionigi G, Rovera F, Boni L. Commentary on transoral access for endoscopic thyroid resection. Surg Endosc 2009;23:454-5. [Crossref] [PubMed]
- Dionigi G, Duran-Poveda M. New approaches in thyroid surgery: is there an increased risk of nerve injury? Ann Surg Oncol 2011;18:S252-3. [Crossref] [PubMed]
- Lee S, Ryu HR, Park JH, et al. Excellence in robotic thyroid surgery: a comparative study of robot-assisted versus conventional endoscopic thyroidectomy in papillary thyroid microcarcinoma patients. Ann Surg 2011;253:1060-6. [Crossref] [PubMed]
- Randolph GW. The Recurrent and Superior Laryngeal Nerves. Springer, 2016.
- Witzel K, Von Rahden B, Kaminski C, et al. Transoral access for endoscopic thyroid resection. Surg Endosc 2008;22:1871-5. [Crossref] [PubMed]
- Dionigi G, Kim HY, Wu CW, et al. Neuromonitoring in endoscopic and robotic thyroidectomy. Updates Surg 2017;69:171-9. [Crossref] [PubMed]
- Xie Q, Wang P, Yan H, et al. Feasibility and Effectiveness of Intraoperative Nerve Monitoring in Total Endoscopic Thyroidectomy for Thyroid Cancer. J Laparoendosc Adv Surg Tech A 2016;26:109-15. [Crossref] [PubMed]
- Wang Y, Yu X, Wang P, et al. Implementation of Intraoperative Neuromonitoring for Transoral Endoscopic Thyroid Surgery: A Preliminary Report. J Laparoendosc Adv Surg Tech A 2016;26:965-71. [Crossref] [PubMed]
- Zhang D, Li F, Wu CW, et al. Percutaneous probe stimulation for intraoperative neuromonitoring in total endoscopic thyroidectomy: a preliminary experience. Head Neck 2017;39:1001-7. [Crossref] [PubMed]
- Lee HY, Lee JY, Dionigi G, et al. The Efficacy of Intraoperative Neuromonitoring During Robotic Thyroidectomy: A Prospective, Randomized Case-Control Evaluation. J Laparoendosc Adv Surg Tech A 2015;25:908-14. [Crossref] [PubMed]
- Sung ES, Lee JC, Kim SH, et al. Development of an Attachable Endoscopic Nerve Stimulator for Intraoperative Neuromonitoring during Endoscopic or Robotic Thyroidectomy. Otolaryngol Head Neck Surg 2018;158:465-8. [Crossref] [PubMed]
- Tae K, Ji YB, Jeong JH, et al. Comparative study of robotic versus endoscopic thyroidectomy by a gasless unilateral axillo-breast or axillary approach. Head Neck 2013;35:477-84. [Crossref] [PubMed]
- Dionigi G, Wu CW, Tufano RP, et al. Monitored transoral endoscopic thyroidectomy via long monopolar stimulation probe. J Vis Surg 2018;4:24. [Crossref] [PubMed]
- Huang TY, Catalfamo A, Wu CW, et al. Neural monitoring in transoral endoscopic thyroidectomy. Ann Thyroid 2018;3:7. [Crossref]
- Kim HY, Chai YJ, Barczynski M, et al. Technical Instructions for Continuous Intraoperative Neural Monitoring in Thyroid Surgery. J Endocr Surg 2018;18:61-78. [Crossref]
- Terris DJ, Anderson SK, Watts TL, et al. Laryngeal nerve monitoring and minimally invasive thyroid surgery: complementary technologies. Arch Otolaryngol Head Neck Surg 2007;133:1254-7. [Crossref] [PubMed]
- Dionigi G, Alesina P, Barczynski M, et al. Recurrent laryngeal nerve injury in video-assisted thyroidectomy: lessons learned from neuromonitoring. Surg Endosc 2012;26:2601-8. [Crossref] [PubMed]
- Dionigi G, Bacuzzi A, Lavazza M, et al. Transoral endoscopic thyroidectomy: preliminary experience in Italy. Updates Surg 2017;69:225-34. [Crossref] [PubMed]
- Dionigi G, Boni L, Rovera F, et al. Neuromonitoring and video-assisted thyroidectomy: a prospective, randomized case-control evaluation. Surg Endosc 2009;23:996-1003. [Crossref] [PubMed]
- Chen HK, Chen CL, Wen KS, et al. Application of transoral continuous intraoperative neuromonitoring in natural orifice transluminal endoscopic surgery for thyroid disease: a preliminary study. Surg Endosc 2018;32:517-25. [Crossref] [PubMed]
- Bacuzzi A, Dralle H, Randolph GW, et al. Safety of Continuous Intraoperative Neuromonitoring (C-IONM) in Thyroid Surgery. World J Surg 2016;40:768-9. [Crossref] [PubMed]
- Rocke DJ, Goldstein DP, de Almeida JR. A Cost-Utility Analysis of Recurrent Laryngeal Nerve Monitoring in the Setting of Total Thyroidectomy. JAMA Otolaryngol Head Neck Surg 2016;142:1199-205. [Crossref] [PubMed]
- Al‐Qurayshi Z, Kandil E, Randolph G. Cost‐effectiveness of intraoperative nerve monitoring in avoidance of bilateral recurrent laryngeal nerve injury in patients undergoing total thyroidectomy. Br J Surg 2017;104:1523-31. [Crossref] [PubMed]
Cite this article as: Ranganath R, Dhillon VK, Russell JO, Tufano RP. The evolution and progress of the application of intraoperative monitoring in endoscopic thyroid surgery. Ann Thyroid 2018;3:30.


