
Evolution and progress of continuous intraoperative neural monitoring
Introduction
Autopsy studies have been instrumental in fostering our understanding of recurrent laryngeal nerve (RLN) anatomy and laryngeal function, laying the groundwork for RLN monitoring (1). With the advent of neck surgery including thyroid resection, Kocher and Lahey pioneered and standardized the meticulous dissection technique, leading the way to modern neck dissection (1,2) (Figure 1).
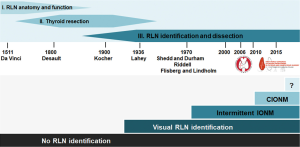
It was in 1969 that Flisberg and Lindholm reported the first successful use of intraoperative nerve monitoring (IONM) during thyroid surgery in man (3). The key elements of dissection they put forth, (I) unambiguous identification of the RLN, and (II) preservation of an intact conduction line between the point of nerve stimulation and the effector vocalis muscle, have become the basic tenet of modern IONM. Another important aspect is the ability of IONM to reliably predict early postoperative vocal cord function (4,5). Although the technique of IONM quickly gained traction, there was still a need to minimize unsupervised monitoring intervals to enable simultaneous dissection and monitoring of RLN function. This unmet need sparked the development of continuous IONM.
As one would expect, many professional associations and societies took a keen interest in the technique of IONM, making it a main topic of scientific meetings. In 2010, the first European Symposium on continuous IONM took place in Leipzig, Germany (6), followed in 2015 by the first World Congress of Intraoperative Neuromonitoring in Krakow, Poland (7), which was initiated and organized by the International Neuromonitoring Study Group (8), an interdisciplinary research group founded in 2007 (Figure 1).
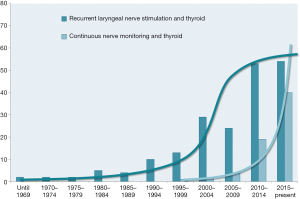
Intermittent and continuous IONM also sparked a lot of interest in clinical and experimental scientific research. A recent Medline query returned proliferating numbers of publications over the years, reaching a climax in the new millennium. Whereas the number of publications on intermittent IONM may be levelling off now, the exponential increase in publications on continuous intraoperative nerve monitoring (CIONM) is continuing unfettered (Figure 2).
Real-time monitoring of RLN function
A double balloon tube including both stimulating and recording surface electrodes was conceived in the mid-1990s for continuous transtracheal stimulation of the RLN (9). Unsolved material and technical issues stalled further development of the double balloon tube prototype, preventing it from progressing beyond animal experiments. It was in 2007 that the innovative concept of a vagus electrode for continuous stimulation and monitoring of the RLN was realized and moved into clinical practice (10). To mount the electrode on the vagus nerve, several centimeter of the vagus nerve must be dissected free of surrounding tissue along the circumference of the nerve. At the same time, another research group developed a less invasive vagus electrode for real-time monitoring (11). To fasten this so-called anchor or T-shape electrode, only a semi-circular dissection around the vagus nerve is needed. Animal experiments revealed that nerve amplitude and latency are the key elements of nerve integrity, paving the way for continuous electromyogram waveform analysis in man. For the first time, it became possible to identify injuries to the RLN caused by traction, compression, or heat, which were heralded by changes in signal amplitude and latency. Characteristically, changes in amplitude (decreases by 40–60%) are greater than changes in latency (increases by 15%), the extent of which typically depends on the severity of the injury. Unknown at the time were those amplitude and latency thresholds that mark the irreversibility of EMG changes even after discontinuation of the causative surgical manoeuvre.
Translating continuous nerve monitoring into clinical practice
Preservation of the RLN is a principal objective of thyroid surgery. Although being used widely, intermittent IONM proved unable to prevent unilateral transient or permanent vocal cord (VC) palsy. With the introduction of the vagal electrode in 2007, the notion of real-time monitoring of RLN function during neck surgery became true. This novel electrode, supported by dedicated software, was successfully employed in 19 patients to monitor RLN function during thyroid surgery. This feasibility study confirmed that continuous RLN monitoring with the vagus electrode was safe, reproducible, and easy to perform. Because it did not interfere with conventional IONM equipment, the vagus electrode proved to be a useful adjunct in the armamentarium of continuous IONM (12).
Almost concomitantly to the introduction of the vagus cuff electrode in 2007, Schneider et al. introduced the anchor electrode into clinical practice (13). No longer requiring dissection all around the nerve, the self-stabilizing anchor electrode is placed on the vagus nerve via a small incision in the anterior surface of the carotid sheath. In a pilot study of 45 consecutive thyroid patients with 78 nerves at risk, the anchor electrode was safe and easy to use, with less nerve dissection needed to attach the electrode.
Another option to support continuous vagus nerve monitoring in real time is the V3 electrode (14), which was used to monitor RLN function in 100 thyroid patients with 188 nerves at risk. In surprising contrast to other researchers, the authors reported that nerve amplitude and latency were unreliable in predicting compromised RLN function during thyroid resection, whereas they agreed that loss of the EMG signal signifies a grave risk to the functional integrity of the RLN.
In recent years, a number of device manufacturers have started to commercialize nerve monitoring equipment with various vagus electrode configurations, EMG displays and/or alarm limits (15). Independent of electrode polarity, shape, size and resilience, all stimulating electrodes represent a compromise between enhanced stability of the EMG signal with fewer electrode dislocations, and greater resilience for better protection of the vagus nerve.
Multiple studies were conducted with a focus on the safety of CIONM (16,17). With rare anecdotal exceptions (18,19), the totality of the clinical evidence argues against an adverse risk inherent in dissection around the vagus nerve or intrinsic to CIONM stimulation (20). Although vagus nerve stimulation causes parasympathetic activity to prevail, this parasympathetic increase was not offset by a compensatory rise of sympathetic activity (21). This increased parasympathetic activity had no appreciable effect on cardiac or hemodynamic parameters, nor on the level of proinflammatory cytokine TNF-α. On balance, CIONM is safe as long as the established standards of IONM are heeded (22).
Impact of CIONM on surgical strategy
Intraoperative damage control
Animal studies on experimental RLN injury showed that direct traction, pressure, or heat applied to the RLN can alter EMG characteristics in a reproducible characteristic manner (11). To facilitate interpretation of clinically relevant quantitative EMG signals, adverse “combined” EMG events with specific concordant changes in both signal amplitude and latency, were defined as >50% decreases in amplitude coupled with >10% increases in latency relative to baseline. When these clinically important EMG thresholds are exceeded, nerve injury appears to evolve with permanent injury included (23) (Figure 3A).
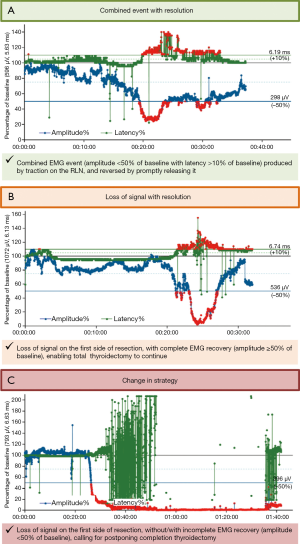
In a proof-of-concept study of 52 patients with 52 nerves at risk, unphysiological traction led to RLN injury. In another series of 102 patients (24), combined EMG events, 73% of which were reversible, yielded a positive predictive value of 33% and a negative predictive value of 97%. Isolated changes in amplitude or latency were not associated with VC palsy. Another larger study of 788 patients with 1,314 nerves at risk who underwent CIONM-guided thyroid surgery found that prompt release of a distressed RLN, evidenced by disappearance of the combined EMG events, does not result in loss of the EMG signal in 80%, based on 63 of 77 nerves at risk (25). This electrophysiological correlate of impending nerve injury can be subdivided into two sequential phases: (I) Amplitude decrease, reflecting reduction of the number of action potential-transmitting nerve cells during and after traction on the nerve. This most early form of EMG alteration in the setting of trauma appears to be most associated with more abrupt and acute onset of the traumatic activity. This is more commonly associated with acute segmental type I injuries; (II) Further amplitude decrease coupled with latency increase as a result of continued nerve traction, indicative of progressive serious nerve damage.
Being cognizant of these signal characteristics allows the surgeon to initiate fast corrective action by immediately releasing the distressed nerve in the first electrophysiological phase, to avoid entering the second electrophysiological phase in which the damage to the RLN is much more severe (17,26).
The International Neuromonitoring Study Group defined loss of the EMG signal (LOS) as complete loss of neural stimulation response when the signal amplitude is <100 µV (5,27). Segmental type 1 and diffuse type 2 nerve injuries differ greatly as to time to loss of the nerve monitoring signal and dynamics of amplitude loss (medians of 2 vs. 156 seconds; P<0.001) (28). Unlike most segmental type 1 injuries in which the nerve monitoring signal is lost all of a sudden, global type 2 injuries are always preceded by combined EMG events. Because segmental LOS is less likely to result in graded partial EMG changes identifiable before definitive injury has occurred, CIONM may not help prevent this type of lesion completely—instead CIONM may aid in mitigating the severity of the damage by causing the surgeon to release the distressed nerve. Advanced training and careful microdissection are keys to minimizing frequency and extent of these nerve injuries. It is noteworthy in this regard that certain anatomic variations such as thin nerves, extralaryngeal branching, and a nerve course anterior to the inferior thyroid artery favor segmental type 1 injuries (23,29). Global LOS type 2, preceded by premonitory combined EMG events, is more easily avoided by immediate reversal of the causative surgical manoeuvre (23,30).
Nerve recovery after segmental or global LOS can be reasonably assumed to take place when the diminished signal amplitude rises above the 100 µV threshold of complete LOS.
In a recent institutional proof-of-concept study of 41 patients with 41 nerves at risk (28), the extent of intraoperative amplitude recovery after LOS, expressed in % of baseline, correlated well with postop VC palsy. Intraoperative signal recovery ≥50% of the initial signal amplitude always signified normal postoperative VC function, whereas signal recovery <50% of nerve baseline amplitude predicted early VC palsy in all patients with segmental type 1 injury and in two-thirds of patients with global type 2 injury. This information is important for surgeons to make up their minds on whether to continue with contralateral thyroidectomy during the same session or whether to embark on a staged approach.
An international multi-center study of 68 patients with 68 nerves at risk that developed rare events of LOS with intraoperative signal recovery (31) confirmed and extended this institutional data. Intraoperative amplitude recovery ≥50% relative to baseline reliably predicted normal early postoperative VC function in all patients after transient segmental LOS type 1 or global LOS type 2. Overall, VC function was compromised in 10 (25%) of 40 patients with transient global LOS type 2, and in 18 (64%) of 28 patients with transient segmental LOS type 1 on the second postoperative day. On receiver-operating characteristics analysis, relative and absolute amplitude recovery of 49% and 455 µV after segmental LOS type 1, and 44% or 253 µV after global LOS type 2 discriminated best between normal and abnormal early postoperative VC function. Remarkably, absolute amplitude thresholds fared no better than relative amplitude thresholds in predicting early postoperative VC function after segmental LOS type 1, and even worse after global LOS type 2. Pragmatically, it is reasonable to apply the same amplitude threshold of ≥50% for both types of LOS (Figure 3B). This single threshold predicts normal early postoperative vocal function accurately after segmental LOS type 1, the more severe form of nerve injury, but may underestimate normal early postoperative vocal function slightly after global LOS type 2, the less serious form of nerve injury (31).
Change in surgical strategy
In clinical practice, it may be prudent to wait after LOS for complete amplitude recovery for at least 15 to 20 minutes before attempting contralateral thyroidectomy (28,31). Usually, nerve function after LOS recovers completely within 6.9–8.0 min in segmental type 1 injury, and within 13.0–15.6 min in global type 2 injury. If the nerve amplitude recovers less than 50% of its baseline, it is not advisable to wait for more than 20 minutes.
When LOS affects the first side of resection in planned bilateral thyroidectomy, three clinical scenarios need to be differentiated to circumvent bilateral RLN palsy (17,32):
- Persistent LOS on this first side of resection (Figure 3C) warrants postponement of contralateral thyroidectomy because as many as 95% of patients with segmental LOS type 1 and 70% of patients with global type 2 have early postoperative VC palsy;
- Incomplete signal recovery (<50% of baseline amplitude) after LOS also requires consideration of staging thyroidectomy because 95% of patients with segmental LOS type 1 and 48% of patients with global LOS type 2 have early postoperative VC palsy;
- Complete signal recovery (≥50% of baseline amplitude) after LOS indicates normal VC function so that it is reasonable to embark on contralateral thyroidectomy during the same session.
Only strict adherence to the International Neuromonitoring Study Group’s L1, V1, R1, R2, V2, L2 concept can maximize the reliability of IONM (5,26). The superior accuracy of CIONM over IIONM, resulting in a sensitivity of 90.9%, specificity of 99.7%, positive predictive value of 88.2%, negative predictive value of 99.8%, and an accuracy of 95% using LOS trouble-shooting algorithms, greatly informs clinical decision-making in the operative suite (25).
Staged thyroidectomy is the logical course of action in experienced hands after recovery of VC function (28,32,33). When the amplitude fails to recover completely, contralateral surgery should be performed only under exceptional circumstances. The afore-mentioned 3 scenarios including attendant clinical ramifications need to be detailed during the informed consent process, empowering the patient to make a personal decision fully reflecting his or her preferences (17,34).
Wider adoption of the concept of incomplete and complete amplitude recovery after LOS may further decrease already low false-positive and false-negative CIONM rates (as compared with IIONM), which currently stand at 0.3% (vs. 0.5%) and 0.25% (vs. 0.6%), as well as the number of unnecessary staged procedures (25,26,28).
Lessons learned from animal models
In the second century AD, Galen was the first to delineate course and function of the RLN (1). Most experimental models have used medium-sized, relatively inexpensive animals, notably canine and porcine species (11,35). As for laryngeal anatomy, function, and size, pigs and dogs are broadly comparable to man, making these species good experimental animal models of human laryngeal physiology (35).
In the past decade, animal studies greatly enhanced our understanding of RLN physiology, which is at the heart of CIONM technology. Important examples include unravelling of EMG characteristics, EMG tube connectivity issues (36), aspects of general anesthesia (37), the safety of electrical nerve stimulation (38), and RLN injury models and preventative strategies.
In the early CIONM era, Schneider et al. (11) evaluated feasibility and safety of a new vagal anchor electrode in a pig model, experimentally determining supramaximum stimulation thresholds. Histopathologic examination of the surgical specimen confirmed that the vagus nerve was intact morphologically while displaying mild epineural edema. These animal studies were extremely important to allay concerns about the safety of repeated nerve stimulation, providing a rational basis for moving an experimental technology into a clinical environment.
Several animal experiments correlated increasing traction and pressure on the RLN with changes in EMG signal characteristics (39-41). These animal models revealed that right and left RLNs can greatly differ in terms of sensitivity to trauma, so that a sensitive nerve is 4.3 times more vulnerable to tensile force than a non-sensitive nerve in the same patient (42). Other animal studies found that the critical temperature of thermal RLN injury depends on the thermal spread from electrocautery and energy-based devices used for hemostasis around the RLN. These findings led to recommendations regarding the safe use of energy-based devices (43-45). In another porcine model, thermal nerve injury caused severe morphological disruption within the endoneurium, whereas traction on the nerve just produced distortion of the nerve sheath (35).
Animal models have propelled, and continue to advance, research and development of IONM: morphological and electrophysiological findings gleaned from animal experiments have immediate clinical relevance to man, in particular when it comes to prevention of RLN injury. CIONM can yield false-positive and false-negative results, which animal studies need to address to push the envelope further.
Recent advances in neck surgery
Children
In an institutional study of 504 children with 755 nerves at risk, CIONM measured nerve electrophysiology more accurately during thyroidectomy than intermittent IONM (46). With CIONM but not IIONM, basal amplitude and latency increased significantly with age and was more pronounced on the left, where the RLN takes an extended path underneath the aortic arch, than on the right, where the RLN wraps around the subclavian artery. With CIONM, amplitudes tended to be greater and latencies shorter than with IIONM, pointing to better connectivity of the former. With CIONM (unlike IIONM), no wound infection, bleeding/hematoma or permanent VC palsy was noted in any child.
When children are older, recording needle electrodes can be exchanged for tube surface electrodes, which generate stronger electrophysiological signals. The unavailability of smaller vagus electrodes and tube surface electrodes hamper extension of CIONM to smaller children. This unmet medical need calls for custom-fit versions of adult electrodes that can be used for children aged 6 years and younger.
Advanced cardiovascular disease
CIONM was shown to be safe in 6 adults with 12 nerves at risk who had second- or third-degree atrioventricular block (20). All 6 patients maintained normal systolic and diastolic blood pressures, heart rate, and peripheral arterial oxygen saturation before, during, and after CIONM. No clinically relevant changes in heart rate or blood pressure, cardiac arrhythmia, or other hemodynamically important events were noted despite careful monitoring of these patients. There was no interference between the biphasic waveform of the vocal muscle electromyogram and the spikes generated by the implanted cardiac pacemakers. Outcomes were uneventful with normal VC and parathyroid gland function.
Endoscopic and robotic thyroid surgery
CIONM has been extended recently to endoscopic and robotic thyroid surgery (47). In a study of 20 patients with 28 nerves at risk, CIONM was applied in conjunction with natural orifice (transoral) transluminal endoscopic surgery for thyroid disease (48). There are two further accounts of CIONM use in single-incision transaxillary robotic thyroidectomy (49). The authors concluded that transaxillary ipsilateral continuous vagus monitoring with the APS vagus electrode was more straightforward than conventional IONM with intermittent manual stimulation from a remote access. Because energy-based devices are essential for endoscopic or robotic thyroid surgery, it is imperative to limit thermal spread as much as possible. There is a need for further research on how to position vagus electrodes on the nerve using current EndoWrist instruments. In a porcine model of transoral endoscopic thyroidectomy vestibular approach (TOETVA) under CIONM, 2 different routes to the vagus nerve were explored, leading to calls for simplification of electrode design and application (50).
Minimally invasive video-assisted thoracoscopic (VATS)
Minimally invasive VATS esophagectomy is another potential application for continuous intraoperative vagus nerve stimulation (51-53). Extended mediastinal lymph node dissection including both RLNs is associated with a 60–70% risk of VC palsy, predisposing the patient to aspiration pneumonia. CIONM may afford more complete node dissection in the neck during esophagectomy, as hinted at by a study describing dissection of more mediastinal lymph node metastases and longer 2-year survival rates with nerve monitoring (54).
Conclusions
CIONM, overcoming the principal limitation of conventional IONM, facilitates faster corrective action by prompt release of distressed nerves. The mastery of this important clinical technology warrants continued mentoring, training, and education, as well as standardization. Present and future research on CIONM is, and will be, directed at improving preservation of voice, breathing, and swallowing, for which postoperative vocal cord function is the best summary measure at the moment. Given the dynamic evolution of CIONM, it may just be a matter of time before the next step in innovation appears in the field.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński, Hui Sun and Xiaoli Liu) for the series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: The series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. H Dralle was remunerated by Medtronic and Inomed for giving lectures on intraoperative nerve monitoring. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kaplan EL, Salti GI, Roncella M, et al. History of the recurrent laryngeal nerve: from Galen to Lahey. World J Surg 2009;33:386-93. [Crossref] [PubMed]
- Lahey F. Routine dissection and demonstration recurrent laryngeal nerve in subtotal thyroidectomy. Surg Gynecol Obstet 1938;66:775-7.
- Flisberg K, Lindholm T. Electrical stimulation of the human recurrent laryngeal nerve during thyroid operation. Acta Otolaryngol Suppl 1969;263:63-7. [PubMed]
- Hermann M, Hellebart C, Freissmuth M. Neuromonitoring in thyroid surgery: prospective evaluation of intraoperative electrophysiological responses for the prediction of recurrent laryngeal nerve injury. Ann Surg 2004;240:9-17. [Crossref] [PubMed]
- Randolph GW, Dralle HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-6. [Crossref] [PubMed]
- Schneider R, Lamade W, Hermann M, et al. Continuous intraoperative neuromonitoring of the recurrent laryngeal nerve in thyroid surgery (CIONM) - Where are we now? An update to the European Symposium of Continuous Neuromonitoring in Thyroid Surgery. Zentralbl Chir 2012;137:88-90. [Crossref] [PubMed]
- Available online: www.ionmworldcongress.com/
- Available online: www.inmsg.org/
- Lamadé W, Meyding-Lamadé U, Hund E, et al. Transtracheal monitoring of the recurrent laryngeal nerve. Prototype of a new tube. Chirurg 1997;68:193-5. [Crossref] [PubMed]
- Lamade W, Ulmer C, Seimer A, et al. A new system for continuous recurrent laryngeal nerve monitoring. Minim Invasive Ther Allied Technol 2007;16:149-54. [Crossref] [PubMed]
- Schneider R, Przybyl J, Pliquett U, et al. A new vagal anchor electrode for real-time monitoring of the recurrent laryngeal nerve. Am J Surg 2010;199:507-514. [Crossref] [PubMed]
- Ulmer C, Koch K, Seimer A, et al. Real-time monitoring of the recurrent laryngeal nerve: an observational clinical trial. Surgery 2008;143:359-65. [Crossref] [PubMed]
- Schneider R, Przybyl J, Hermann M, et al. A new anchor electrode design for continuous neuromonitoring of the recurrent laryngeal nerve by vagal nerve stimulations. Langenbecks Arch Surg 2009;394:903-10. [Crossref] [PubMed]
- Jonas J. Continuous vagal nerve stimulation for recurrent laryngeal nerve protection in thyroid surgery. Eur Surg Res 2010;44:185-91. [Crossref] [PubMed]
- Schneider R, Lorenz K, Machens A, et al. Continuous intraoperative neuromonitoring (CIONM) of the recurrent laryngeal nerve. In: Randolph GW (ed.). Switzerland: The Recurrent and Superior Laryngeal Nerves, Springer International, 2016:169-83.
- Dionigi G, Chiang FY, Dralle H, et al. Safety of neural monitoring in thyroid surgery. Int J Surg 2013;11:S120-6. [Crossref] [PubMed]
- Schneider R, Randolph GW, Barczynski M, et al. Continuous intraoperative neural monitoring of the recurrent nerves in thyroid surgery: a quantum leap in technology. Gland Surg 2016;5:607-16. [Crossref] [PubMed]
- Terris DJ, Chaung K, Duke WS. Continuous vagal nerve monitoring is dangerous and should not routinely be done during thyroid surgery. World J Surg 2015;39:2471-6. [Crossref] [PubMed]
- Brauckhoff K, Vik R, Sandvik L, et al. Impact of EMG changes in continuous vagal nerve monitoring in high-risk endocrine neck surgery. World J Surg 2016;40:672-80. [Crossref] [PubMed]
- Schneider R, Machens A, Bucher M, et al. Continuous intraoperative monitoring of vagus and recurrent laryngeal nerve function in patients with advanced atrioventricular block. Langenbecks Arch Surg 2016;401:551-6. [Crossref] [PubMed]
- Ulmer C, Friedrich C, Kohler A, et al. Impact of continuous intraoperative neuromonitoring on autonomic nervous system during thyroid surgery. Head Neck 2011;33:976-84. [Crossref] [PubMed]
- Friedrich C, Ulmer C, Rieber F, et al. Safety analysis of vagal nerve stimulation for continuous nerve monitoring during thyroid surgery. Laryngoscope 2012;122:1979-87. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [Crossref] [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents RLN paralysis by revealing initial EMG changes of impending neuropraxic injury: A prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. Br J Surg 2015;102:1380-7. [Crossref] [PubMed]
- Schneider R, Machens A, Randolph GW, et al. Opportunities and challenges of intermittent and continuous intraoperative neural monitoring in thyroid surgery. Gland Surg 2017;6:537-45. [Crossref] [PubMed]
- Schneider R, Randolph G, Dionigi G, et al. Prospective study of vocal fold function after loss of the neuromonitoring signal in thyroid surgery: The International Neural Monitoring Study Group’s POLT Study. Laryngoscope 2016;126:1260-6. [Crossref] [PubMed]
- Schneider R, Sekulla C, Machens A, et al. Dynamics of loss and recovery of the nerve monitoring signal during thyroidectomy predict early postoperative vocal fold function. Head Neck 2016;38:E1144-51. [Crossref] [PubMed]
- Wu CW, Wang MH, Chen CC, et al. Loss of signal in recurrent nerve neuromonitoring: causes and management. Gland Surg 2015;4:19-26. [PubMed]
- Schneider R, Bures C, Lorenz K, et al. Evolution of nerve injury with unexpected EMG signal recovery in thyroid surgery using continuous intraoperative neuromonitoring. World J Surg 2013;37:364-8. [Crossref] [PubMed]
- Schneider R, Randolph G, Dionigi G, et al. Prediction of postoperative vocal fold function after intraoperative recovery of loss of signal. The International Neuromonitoring Study Group’s PREC Study. Laryngoscope 2018. [Epub ahead of print].
- Schneider R, Lorenz K, Sekulla C, et al. Surgical strategy during intended total thyroidectomy after loss of EMG signal on the first side of resection. Chirurg 2015;86:154-63. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 2012;99:1089-95. [Crossref] [PubMed]
- Dralle H, Schneider R, Lorenz K, et al. Vocal cord paralysis after thyroid surgery: Current medicolegal aspects of intraoperative neuromonitoring. Chirurg 2015;86:698-706. [Crossref] [PubMed]
- Wu CW, Randolph GW, Lu IC, et al. Intraoperative neural monitoring in thyroid surgery: lessons learned from animal studies. Gland Surg 2016;5:473-80. [Crossref] [PubMed]
- Kim HY, Tufano RP, Randolph G, et al. Impact of positional changes in neural monitoring endotracheal tube on amplitude and latency of electromyographic response in monitored thyroid surgery: Results from the Porcine Experiment. Head Neck 2016;38:E1004-8. [Crossref] [PubMed]
- Lu IC, Wu CW, Chang PY, et al. Reversal of rocuronium-induced neuromuscular blockade by sugammadex allows for optimization of neural monitoring of the recurrent laryngeal nerve. Laryngoscope 2016;126:1014-9. [Crossref] [PubMed]
- Xiaoli L, Wu CW, Kim HY, et al. Gastric acid secretion and gastrin release during continuous vagal neuromonitoring in thyroid surgery. Langenbecks Arch Surg 2017;402:265-72. [Crossref] [PubMed]
- Lee HY, Cho YG, You JY, et al. Traction injury of the recurrent laryngeal nerve: Results of continuous intraoperative neuromonitoring in a swine model. Head Neck 2016;38:582-8. [Crossref] [PubMed]
- Brauckhoff K, Aas T, Biermann M, et al. EMG changes during continuous intraoperative neuromonitoring with sustained recurrent laryngeal nerve traction in a porcine model. Langenbecks Arch Surg 2017;402:675-81. [Crossref] [PubMed]
- Brauckhoff K, Svendsen ØS, Stangeland L, et al. Injury mechanisms and electromyographic changes after injury of the recurrent laryngeal nerve: Experiments in a porcine model. Head Neck 2018;40:274-82. [Crossref] [PubMed]
- Lamadé W, Béchu M, Lauzana E, et al. The weepy nerve-different sensitivity of left and right recurrent laryngeal nerves under tensile stress in a porcine model. Langenbecks Arch Surg 2016;401:983-90. [Crossref] [PubMed]
- Lin YC, Dionigi G, Randolph GW, et al. Electrophysiologic monitoring correlates of recurrent laryngeal nerve heat thermal injury in a porcine model. Laryngoscope 2015;125:E283-90. [Crossref] [PubMed]
- Kwak HY, Dionigi G, Kim D, et al. Thermal injury of the recurrent laryngeal nerve by THUNDERBEAT during thyroid surgery: findings from continuous intraoperative neuromonitoring in a porcine model. J Surg Res 2016;200:177-82. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Kim HY, et al. Safety of LigaSure in recurrent laryngeal nerve dissection-porcine model using continuous monitoring. Laryngoscope 2017;127:1724-9. [Crossref] [PubMed]
- Schneider R, Machens A, Sekulla C, et al. Twenty-year experience of paediatric thyroid surgery using intraoperative nerve monitoring. Br J Surg 2018;105:996-1005. [Crossref] [PubMed]
- Dionigi G, Chiang FY, Hui S, et al. Continuous intraoperative neuromonitoring (C-IONM) technique with the Automatic Periodic Stimulating (APS) accessory for conventional and endoscopic thyroid surgery. Surg Technol Int 2015;26:101-14. [PubMed]
- Chen HK, Chen CL, Wen KS, et al. Application of transoral continuous intraoperative neuromonitoring in natural orifice transluminal endoscopic surgery for thyroid disease: a preliminary study. Surg Endosc 2018;32:517-25. [Crossref] [PubMed]
- Lörincz BB, Möckelmann N, Busch CJ, et al. Automatic periodic stimulation of the vagus nerve during single-incision transaxillary robotic thyroidectomy: Feasibility, safety, and first cases. Head Neck 2016;38:482-5. [Crossref] [PubMed]
- Zhang D, Li S, Dionigi G, et al. Feasibility of continuous intraoperative neural monitoring during transoral endoscopic thyroidectomy vestibular approach in a porcine model. J Laparoendosc Adv Surg Tech A 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Tsang RK, Law S. Adaptation of continuous intraoperative vagus nerve stimulation for monitoring of recurrent laryngeal nerve during minimally invasive esophagectomy. World J Surg 2016;40:137-41. [Crossref] [PubMed]
- Wong I, Tong DKH, Tsang RKY, et al. Continuous intraoperative vagus nerve stimulation for monitoring of recurrent laryngeal nerve during minimally invasive esophagectomy. J Vis Surg 2017;3:9. [Crossref] [PubMed]
- Deguchi T, Ikeda Y, Niimi M, et al. Continuous intraoperative neuromonitoring study using pigs for the prevention of mechanical recurrent laryngeal nerve injury in esophageal surgery. Surg Innov 2017;24:115-21. [Crossref] [PubMed]
- Zhong D, Zhou Y, Li Y, et al. Intraoperative recurrent laryngeal nerve monitoring: a useful method for patients with esophageal cancer. Dis Esophagus 2014;27:444-51. [Crossref] [PubMed]
Cite this article as: Schneider R, Machens A, Randolph G, Kamani D, Liddy W, Lorenz K, Dralle H. Evolution and progress of continuous intraoperative neural monitoring. Ann Thyroid 2018;3:29.


