Postpartum thyroid disorders
Introduction
Several changes in the immune system occur during pregnancy (1), of which prominent ones are the localized protective mechanisms of the placenta and systemic immune responses (2,3). Immune responses are categorized as cell mediated reactions by T helper1 (Th1) and humoral responses by T helper2 (Th2). Since Th1 cytokines can have adverse effects on the fetus, a shift in T-cell physiology from Th1 to Th2 immune responses occurs in pregnant women (4,5). Alterations in cellular immunity have also been reported during pregnancy; although during the first trimester, helper/suppressor ratio (CD4/CD8) is increased, it declines in the two following trimesters (6).
Specific immune responses mentioned above are slowly lost during the postpartum period. Prior to return to the pre-pregnancy state, there is a “period of exacerbation” of immune reactivity, 3 to 12 months following delivery (1,7); this over-reactivity is accompanied by beginning, relapse or worsening of autoimmune thyroid disorders. Although all disorders of thyroid may occur within 12 months of birth, two major presentations of thyroid dysfunction in this period are postpartum thyroiditis (PPT) and Graves’ disease (GD).
The aim of this article is to review causes, predictors, clinical presentations, diagnosis and management of PPT and postpartum thyrotoxicosis.
PPT
Postpartum thyroid dysfunction (PPTD) includes a category of autoimmune disorders, distinguished by postpartum occurrence of transient or permanent thyroid dysfunction. PPT is an autoimmune disease and worldwide epidemiological studies have documented a wide range of PPT prevalence, 1.1% to 11.4% (8-15). Differences in the rate of PPT are probably related to variations in ethnicity, geographical locations, and methods or numbers of follow-up visits in the first year post-delivery (16-18). The mean prevalence is approximately 7% (19). PPT is more prevalent in women with other autoimmune disorders and particularly in women with type 1 diabetes in whom its prevalence may reach as high as 18–25% (20-22).
Predictors of PPT
This disease is related to immunologic disturbances that occur in pregnancy and postpartum period. There is a distinct association of PPT with elevated maternal serum thyroid peroxidase antibodies (TPOAb) (23). It is reported that half of TPOAb positive women will eventually be affected by PPT (1,24) and a large number of these patients will develop permanent hypothyroidism. In one study, permanent hypothyroidism occurred in 22 out of 48 (46%) of TPOAb positive with PPT and 1 out of 70 (1.4%) TPOAb negative women without PPT (12). In the largest cohort of women with PPT, 63% of 148 women became permanently hypothyroid 1–120 months after levothyroxine withdrawal (12). The risk of PPT in TPOAb-negative women is approximately one tenth to one hundredth of that in TPOAb-positive women (23). Previous reports have found a relationship between immunogenetic factors such as HLA-DR3, -DR-4 and -DR-5, with thyroid-specific autoimmune diseases (23,24).
Other factors associated with the PPT development are age, multiparty and a previous history of spontaneous abortion (25,26).
Clinical presentation
PPT may present as hyperthyroidism, hypothyroidism or may have both clinical presentations (27). The hyperthyroid phase most commonly occurs at 3 months (range between 1 to 6 months) postpartum and lasts only 1–2 months. Postpartum GD usually occurs between 4–7 months postpartum. In the thyrotoxic phase of PPT, the symptoms and signs of hyperthyroidism are milder than during thyrotoxicosis due to GD. The differential diagnosis of these two diseases has been included in the second part of this article.
It has been reported that in 30% of women with PPT, the thyrotoxic phase of the disease is not clinically detected because of lack of symptoms of hyperthyroidism; 20–30% of PPT patients have only hyperthyroid findings and other patients may develop only signs and symptoms of hypothyroidism (approximately 40–50%) (27).
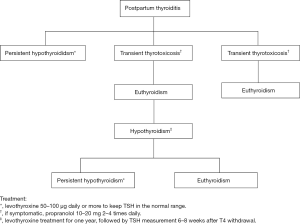
The hypothyroid phase of PPT most commonly occurs at 6 months (range between 3–8 months) postpartum, preceded by a hyperthyroid phase in 25–35% of cases (28,29). The most common presentations of the hypothyroidism phase of PPT are constipation, fatigue, poor memory, loss of concentration and depression (15,23,30). Figure 1 indicates thyroid status in most common types of PPT.
Postpartum depression (PPD) occurs in 10% of non-selected women (31). The possible relationship between PPD and PPT has long been debated. Hypothyroidism may decrease 5-hydroxytrytamine neurotransmission (32) and cytokines such as IL-1 and IL-6released during thyroid autoimmune reactions may interact with central neurotransmission and initiate depression (33,34). Some studies have shown the association of thyroid antibodies with PPD whereas others have not (24,35-38).
Treatment
In the thyrotoxic phase of PPT, antithyroid drug use is not indicated. Propranolol should be employed for those with fatigue, palpitation, heat intolerance and nervousness. This should be discontinued after 1–2 months, when this phase has subsided.
Levothyroxine should be given in symptomatic women during the hypothyroid phase of PPT (39). Asymptomatic females with TSH levels <10 mU/L who are not planning to get pregnant in the next one year, do not require levothyroxine treatment. They should be re-evaluated in 4-8 weeks. All those with TSH >10 mU/L and subclinical hypothyroid women who are attempting to become pregnant should be treated with levothyroxine.
Further management
Postpartum thyroid dysfunction may be transient or permanent (27,39). Majority of women attain euthyroidism within one year after diagnosis. However, abnormalities in thyroid ultrasonography and/or iodine percolate discharge test may persist following recovery from hypothyroidism (40,41). Due to underlying autoimmune thyroid disease, 20–64% of women develop hypothyroidism during follow-up (12,26).
Long-term investigations have been conducted among females with PPT. Reported prevalences of recurrent or permanent hypothyroidism have been 12–23% in 3–3.5 years, 24.5% after 6.6 years and 44% after 8.7 years (11,12,27,41,42). In the largest cohort of PPT patients followed after stopping levothyroxine, hypothyroidism occurred in 63% of 148 women with overt hypothyroidism (12). In the only study that has separated outcomes in subclinical hypothyroid cases, 53% of women who initially had subclinical hypothyroidism became hypothyroid, for an average of 23 months, following withdrawal of levothyroxine (43). It has been reported that patients with PPTD who remained euthyroid after LT4 withdrawal, had significantly higher TSH levels versus the control group and 73.3% had elevated TPOAb levels (44); hence, if assessed later, many of these patients could be affected by thyroid failure.
Recommendations
Both the American Thyroid Association (ATA) and the Endocrine Society have published recommendations (28,29) as follows:
- There are insufficient data recommending universal screening for PPT;
- Women with positive TPOAb, those with previous history of PPT and females with type 1 diabetes should have a serum TSH measurement 3–6 months after delivery;
- Women with PPT who have TSH >10 mU/L and those with subclinical hypothyroidism planning a subsequent pregnancy should be treated with levothyroxine;
- All women with PPT with either overt or subclinical hypothyroidism, treated or untreated with levothyroxine should be followed for several years; even those with euthyroidism without levothyroxine therapy need monitoring for occurrence of hypothyroidism;
- Although the association of PPT and PPD is controversial, women with PPD should be screened for hypothyroidism, because hypothyroidism is a potentially reversible cause of depression.
In conclusion, high risk postpartum women should be screened for PPT and individuals with both subclinical and overt PPT should be followed for long durations. Timely diagnosis of the disease and appropriate clinical management and follow-up are vital and improve the quality of life of both mothers and their infants.
Postpartum thyrotoxicosis
Hyperthyroidism occurs more frequently during the postpartum period than at any other time in women. The incidence of postpartum GD is 0.2% (28), whereas that of hyperthyroidism due to postpartum thyroidits is 10–20 times more than GD in other periods of a woman’s life (9,45).
Pathophysiology
The immune suppression present during pregnancy is lost after delivery; however, its return to normal following a period of exacerbation of autoimmunity occurs between 3–12 months postpartum and can cause the onset, relapse or exacerbation of autoimmune thyroid diseases (44-46). Therefore, women with a history of GD, in particular those with hyperthyroidism in the first trimester of pregnancy are more likely to have a relapse or exacerbation of symptoms of hyperthyroidism.
In GD, TSH receptor antibodies (TRAb) stimulate thyroid hormone production and secretion by binding to TSH receptors of thyroid cells. Like other autoimmune reactions, the TRAb effect is also suppressed during pregnancy, which explains the improvement or remission of hyperthyroidism in some patients in the third trimester of pregnancy. However, this autoimmune suppression ends a few weeks after delivery when the serum concentration of TRAb may increase, leading to stimulation of thyroid function.
One of the features of this disease is hyperthyroidism which may occur in isolation, or most often prior to a period of hypothyroidism (1). Destruction of thyroid cells by lymphocytes may cause an initial release of thyroid hormones, followed by a transient or permanent fall in hormone synthesis and release (47). Increase in serum thyroid proximate antibodies (TPOAb) is also detectable in GD.
Clinical presentation
There might be some difficulty in differentiating symptoms of hyperthyroidism from the normal puerperium experience. Excessive fatigue, feeling sleepiness and manic behavior may be reported by hyperthyroid women during the postpartum period. Fatigue and mood disturbance may simulate a clinical picture of PPD (48). Other symptoms of hyperthyroidism such as irritability and palpitation, heat intolerance, increased sweating and mood swings may be present in some women with postpartum hyperthyroidism, although they overlap with normal postpartum symptoms (49).
In postpartum Graves’ hyperthyroid patients, signs such as tachycardia, loss of weight, tremor, thyroid eye disease and diffuse goiter may be present, while in patients in the hyperthyroidism phase of PPT, the symptoms and signs are milder than those of Graves’ hyperthyroidism (9,45).
Laboratory diagnosis
Combinations of a low or undetectable serum TSH and a high serum free T4 (FT4) or FT4 index and/or T3 confirm the diagnosis of thyrotoxicosis (50). Low serum TSH may be seen in old age, early pregnancy, central hypothyroidism, and postpartum hypophysitis and non-thyroidal illness, none of which may be considered in the clinical setting of these postpartum women. In the postpartum period, thyrotoxicosis may present as subclinical hyperthyroidism with a low or undetectable serum TSH and normal FT4 and free T3 concentrations. Serum FT4 or FT4 index may also be normal in patients with T3 thyrotoxicosis and in those with a low serum thyroxine binding globulin concentration. An elevated FT4 should also be considered in those receiving amiodarone, oral cholecystographic agents, and high doses of propranolol, in amphetamine abuse or in patients with non-thyroidal illnesses and familial dysalbuminemic hyperthyroxinemia (44).
Causes of postpartum thyrotoxicosis
Since different etiologies of thyrotoxicosis in the postpartum period necessitate different therapies, it is vital that the diagnosis of the primary cause of the condition be clarified. Essential features of the main etiologies of thyrotoxicosis during the postpartum period are given in Table 1.
Table 1
| Characteristic | Graves’ disease | Postpartum thyroiditis | Painless sporadic thyroiditis | Painful subacute thyroiditis |
|---|---|---|---|---|
| Frequency | Common | Most common | Rare | Rare |
| Timing | 4–7 months postpartum | 2–6 months postpartum | Any time | Any time (seasonal) |
| Symptoms | Hyperthyroidism | Mild hyperthyroidism | None | Fever, malaise |
| Signs | Goiter, exophthalmos hyperthyroidism | Goiter | Goiter | Tender goiter, mild hyperthyroidism |
| Etiology | Autoimmune | Autoimmune | Autoimmune | Unknown |
| Erythrocyte sedimentation rate | Normal | Normal | Normal | High |
| Radioiodine uptake | High | Low | Low | Low |
| Triiodothyronine/thyroxine ratio | High | Low | Low | Variable |
| Thyroid peroxidase antibodies | High | High | High | Low or absent |
| Thyrotropin receptor-stimulating antibodies | Present | Absent | Absent | Absent |
*, rare etiologies are toxic nodular goiter, iodine-induced and iatrogenic thyrotoxicosis.
GD
GD presenting during the first year after delivery is called postpartum GD. The postpartum period is associated with a greater frequency of the onset, exacerbation, and relapse of Graves’ hyperthyroidism. The occurrence of postpartum GD has been reported to range between 12% and 40% in women of childbearing age (51). Patients in clinical remission during pregnancy are prone to postpartum relapse. A dramatic rise occurs in serum TRAbs (52) during this period. Clinical symptoms and signs are usually prominent. There are increases in T3, T4 and FT3/FT4 ratio.
PPT
Women who develop PPT have mild symptoms of hyperthyroidism or may be asymptomatic. They have a higher prevalence of positive TPOAb, lesser changes in their T-cell helper/suppressor ratio, and exaggerated thyroid autoantibody responses during pregnancy and postpartum (6). The thyrotoxic phase usually occurs 2–6 months after delivery and is self-limited; women become euthyroid or hypothyroid during the following postpartum months, although GD may coexist with PPT (53).
Subacute painful thyroiditis
This disorder may rarely occur during the postpartum period and is not related to autoimmune re-activation of this period. The representative clinical features are pain in the region of the thyroid and constitutional symptoms. Serum TSH is usually suppressed and FT4 and free T3 may mildly be increased. Elevated erythrocyte sedimentation rate and a low radioiodine thyroid uptake (RAIU) establish the diagnosis of subacute thyroiditis (54).
Painless sporadic thyroiditis
Clinical and laboratory characteristics and management of this rare autoimmune disorder are the same as those of the PPT. Patients with painless sporadic thyroiditis have no or very mild symptoms and a small, diffuse goiter is palpable in half of them (54).
Other forms of thyrotoxicosis
Iatrogenic thyrotoxicosis may very rarely be noted in the differential diagnosis of thyrotoxicosis in a young mother. The diagnosis is confirmed by absence of goiter, a low RAIU and low serum thyroglobulin. Iodine-induced thyrotoxicosis and toxic nodular goiter may also occur in the postpartum period.
Differential diagnosis
Diagnosis of the etiology of hyperthyroidism may be possible based only on history and physical examination. Findings of thyroid eye disease in GD and a painful thyroid in subacute thyroiditis present the most prominent clinical findings.
The major task is differentiation of GD from the thyrotoxic phase of PPT (55); goiter usually occurs in both diseases but is often more prominent in the former. The RAIU is always low in PPT, elevated or normal in GD; TRAbs are positive in GD and negative in PPT.
Serum triiodothyronine/thyroxine ratio and FT3/FT4 ratio are increased in GD and decreased in most patients with destructive thyroiditis, iatrogenic, or iodine-induced thyrotoxicosis. The RAIU is increased in GD where hyperthyroidism is caused by excessive thyroid hormone synthesis and release; however, various types of thyroiditis are accompanied by a low RAIU because inflammatory changes in the thyroid result in suppressed TSH and an inability to iodine concentration (56). Low thyroid RAIU is also detected in exogenous thyroid hormone ingestion, strauma ovarii, and iodine-induced thyrotoxicosis.
In the PPT, increase in serum thyroglobulin concentrations precedes the onset of thyrotoxicosis, while in GD, an elevated thyroglobulin is accompanied by increase in FT4 and free T3 (28). The presence of systematic symptoms, thyroid pain, increased erythrocyte sedimentation rate and C-reactive protein in subacute thyroiditis differentiate this disease from PPT; in addition, TPOAb titers are elevated in the latter (57,58). Another discriminating factor is that increased thyroid blood flow in Doppler ultrasonography is seen in GD, while it is low in PPT (59).
Treatment
Patients in the thyrotoxic phase of PPT have relatively few symptoms lasting only a few weeks and do not need any treatment. Antithyroid drugs are not indicated because the etiology of hyperthyroidism is excessive release of thyroid hormones and biosynthesis is not increased (60). Sodium ipodate remarkably inhibits the peripheral deiodination of T4 to generate T3 and may be administered 500 mg daily in severe thyrotoxicosis caused by destruction-induced thyrotoxicosis (61).
Radioiodine administration is one therapeutic modality for postpartum GD; however, infant care for up to 7 days or longer according to radiation safety instructions may be very difficult for the mother; likewise, radioiodine treatment is contraindicated in the breast-feeding mother (45).
Antithyroid drugs are the treatment of choice of GD during the postpartum period (46,47). The doses, duration, and follow-up management of hyperthyroidism with antithyroid drugs are the same as those for patients with GD who are not in the postpartum period. Continuing antithyroid drugs for 12–18 months followed by appropriate monitoring to detect any relapse is reasonable and recommended.
If the mother is not breast feeding, radioiodine therapy becomes the treatment of choice when there is contraindication for use of methimazole (MMI) or when side effects are encountered during medical treatment. For the occasional patient who develops side effects of antithyroid drugs and avoids radioiodine treatment, subtotal thyroidectomy would be advisable, following careful control of hyperthyroidism by antithyroid drug (28,29). Subacute painful thyroiditis should be treated with β-blockers. In the presence of moderate or severe signs and symptoms, nonsteroidalanti-inflammatory agents or prednisolone may also be considered.
Breast-feeding
Breast-feeding women with hyperthyroidism require special considerations both in diagnosis and management of thyrotoxicosis.
Diagnosis
Radioiodine should not be given to breast-feeding women, because it is secreted in the milk. Ingestion of 5–10 µCi doses of 123I and 131I for thyroid uptake test requires discontinuation of nursing for 2 days and few weeks, respectively (62). Use of thyroid RAIU test for the differential diagnosis of thyrotoxicosis must be limited; in special cases, 123I should be used because of its extremely short physical half-life of 13 hours, compared to the 8-dayhalf-life of 131I. In postpartum females who present with clinical and biochemical findings of thyrotoxicosis, if the T3/T4 ratio is >20 and the erythrocyte sedimentation rate is normal, the diagnosis of GD is indicated; confirmation of diagnosis may be made by measurement of TRAb.
Treatment
After a long-lasting debate it is now clear that the thyroid function and physical and intellectual development of children, breastfed by thyrotoxic mothers on anti-thyroid drugs remains unaffected (63). It has been shown that treatment of breast-feeding mothers for up to one year (daily dose of 20 and 30 mg MMI) at initiation of treatment and decrease to maintenance dose by the titration method do not cause alterations in the thyroid function of their infants (63-65). Furthermore, thyroid function tests and physical and intellectual growth of these children are all normal and comparable to children who were breastfed by normal non-thyrotoxic mothers (64,66). It is estimated that peak concentration of milk MMI after a dose of 40 mg is 0.72±0.07 µg/mL and that following ingestion of a single 20 mg dose of MMI by mother, the breastfed infant receives approximately 50 µg MMI (7 µg/kg for a 5-month-old infant). Serum MMI concentration of breastfed infants whose mothers received 20–30 mg MMI daily was less than 0.03 µg/mL (44,64). Based on the above findings, MMI concentration is too low to have any side effects. For more safety, it is recommended to breast-feed the infant before ingestion of MMI (44).
It is concluded that clinicians should carefully proceed with proper diagnosis and treatment of women with postpartum hyperthyroidism and ensure that correct management will improve the quality of life of mothers and their infants.
Acknowledgments
The authors wish to acknowledge Ms. Niloofar Shiva and Dr. Hengameh Abdi for critical editing of English grammar and syntax of the manuscript and Ms. Tahereh Fakhimi for typing the manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for the series “Thyroid and Pregnancy”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.04.04). The series “Thyroid and Pregnancy” was commissioned by the editorial office without any funding or sponsorship. Fereidoun Azizi served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Thyroid from June 2017 to May 2019. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Amino N, Tada H, Hidaka Y. Autoimmune thyroid disease and pregnancy. J Endocrinol Invest 1996;19:59-70. [Crossref] [PubMed]
- Carosella ED, Dausset J, Kirszenbaum M. HLA-G revisited. Immunol Today 1996;17:407-9. [Crossref] [PubMed]
- King A, Loke YW, Chaouat G. NK cells and reproduction. Immunol Today 1997;18:64-6. [Crossref] [PubMed]
- Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353-6. [Crossref] [PubMed]
- Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol 1993;23:3043-9. [Crossref] [PubMed]
- Stagnaro-Green A, Roman SH, Cobin RH, et al. A prospective study of lymphocyte-initiated immunosuppression in normal pregnancy: evidence of a T-cell etiology for postpartum thyroid dysfunction. J Clin Endocrinol Metab 1992;74:645-53. [PubMed]
- Lazarus JH, Ludgate ME. Prevention and treatment of postpartum Graves' disease. Baillieres Clin Endocrinol Metab 1997;11:549-60. [Crossref] [PubMed]
- Amino N, Mori H, Iwatani Y, et al. High prevalence of transient post-partum thyrotoxicosis and hypothyroidism. N Engl J Med 1982;306:849-52. [Crossref] [PubMed]
- Freeman R, Rosen H, Thysen B. Incidence of thyroid dysfunction in an unselected postpartum population. Arch Intern Med 1986;146:1361-4. [Crossref] [PubMed]
- Lervang HH, Pryds O, Ostergaard Kristensen HP. Thyroid dysfunction after delivery: incidence and clinical course. Acta Med Scand 1987;222:369-74. [Crossref] [PubMed]
- Nikolai TF, Turney SL, Roberts RC. Postpartum lymphocytic thyroiditis. Prevalence, clinical course, and long-term follow-up. Arch Intern Med 1987;147:221-4. [Crossref] [PubMed]
- Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab 2000;85:71-5. [Crossref] [PubMed]
- Roti E, Bianconi L, Gardini E, et al. Postpartum thyroid dysfunction in an Italian population residing in an area of mild iodine deficiency. J Endocrinol Invest 1991;14:669-74. [Crossref] [PubMed]
- Shahbazian HB, Sarvghadi F, Azizi F. Prevalence and characteristics of postpartum thyroid dysfunction in Tehran. Eur J Endocrinol 2001;145:397-401. [Crossref] [PubMed]
- Walfish PG, Meyerson J, Provias JP, et al. Prevalence and characteristics of post-partum thyroid dysfunction: results of a survey from Toronto, Canada. J Endocrinol Invest 1992;15:265-72. [Crossref] [PubMed]
- Davies TF. The thyroid immunology of the postpartum period. Thyroid 1999;9:675-84. [Crossref] [PubMed]
- Lazarus JH. Prediction of postpartum thyroiditis. Eur J Endocrinol 1998;139:12-3. [Crossref] [PubMed]
- Roti E, Uberti E. Post-partum thyroiditis--a clinical update. Eur J Endocrinol 2002;146:275-9. [Crossref] [PubMed]
- Stagnaro-Green A. Postpartum thyroiditis. Best Pract Res Clin Endocrinol Metab 2004;18:303-16. [Crossref] [PubMed]
- Alvarez-Marfany M, Roman SH, Drexler AJ, et al. Long-term prospective study of postpartum thyroid dysfunction in women with insulin dependent diabetes mellitus. J Clin Endocrinol Metab 1994;79:10-6. [PubMed]
- Benvenga S, Pintaudi B, Vita R, et al. Serum thyroid hormone autoantibodies in type 1 diabetes mellitus. J Clin Endocrinol Metab 2015;100:1870-8. [Crossref] [PubMed]
- Di Bari F, Granese R, Le Donne M, et al. Autoimmune Abnormalities of Postpartum Thyroid Diseases. Front Endocrinol (Lausanne) 2017;8:166. [Crossref] [PubMed]
- Amino N, Tada H, Hidaka Y. Postpartum autoimmune thyroid syndrome: a model of aggravation of autoimmune disease. Thyroid 1999;9:705-13. [Crossref] [PubMed]
- Harris B, Othman S, Davies JA, et al. Association between postpartum thyroid dysfunction and thyroid antibodies and depression. BMJ 1992;305:152-6. [Crossref] [PubMed]
- Azizi F. Age as a predictor of recurrent hypothyroidism in patients with post-partum thyroid dysfunction. J Endocrinol Invest 2004;27:996-1002. [Crossref] [PubMed]
- Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev 2001;22:605-30. [Crossref] [PubMed]
- Othman S, Phillips DI, Parkes AB, et al. A long-term follow-up of postpartum thyroiditis. Clin Endocrinol (Oxf) 1990;32:559-64. [Crossref] [PubMed]
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012;97:2543-65. [Crossref] [PubMed]
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017;27:315-89. [Crossref] [PubMed]
- Lucas A, Pizarro E, Granada ML, et al. Postpartum thyroiditis: epidemiology and clinical evolution in a nonselected population. Thyroid 2000;10:71-7. [Crossref] [PubMed]
- Stamp GE, Crowther CA. Postnatal depression: a South Australian prospective survey. Aust N Z J Obstet Gynaecol 1994;34:164-7. [Crossref] [PubMed]
- Cleare AJ, McGregor A, Chambers SM, et al. Thyroxine replacement increases central 5-hydroxytryptamine activity and reduces depressive symptoms in hypothyroidism. Neuroendocrinology 1996;64:65-9. [Crossref] [PubMed]
- Hall R. Pregnancy and autoimmune endocrine disease. Baillieres Clin Endocrinol Metab 1995;9:137-55. [Crossref] [PubMed]
- Le Donne M, Mento C, Settineri S, et al. Postpartum Mood Disorders and Thyroid Autoimmunity. Front Endocrinol (Lausanne) 2017;8:91. [Crossref] [PubMed]
- Kuijpens JL, Vader HL, Drexhage HA, et al. Thyroid peroxidase antibodies during gestation are a marker for subsequent depression postpartum. Eur J Endocrinol 2001;145:579-84. [Crossref] [PubMed]
- Pop VJ, de Rooy HA, Vader HL, et al. Microsomal antibodies during gestation in relation to postpartum thyroid dysfunction and depression. Acta Endocrinol (Copenh) 1993;129:26-30. [PubMed]
- Kent GN, Stuckey BG, Allen JR, et al. Postpartum thyroid dysfunction: clinical assessment and relationship to psychiatric affective morbidity. Clin Endocrinol (Oxf) 1999;51:429-38. [Crossref] [PubMed]
- Lucas A, Pizarro E, Granada ML, et al. Postpartum thyroid dysfunction and postpartum depression: are they two linked disorders? Clin Endocrinol (Oxf) 2001;55:809-14. [Crossref] [PubMed]
- Stagnaro-Green A. Clinical review 152: Postpartum thyroiditis. J Clin Endocrinol Metab 2002;87:4042-7. [Crossref] [PubMed]
- Adams H, Jones MC, Othman S, et al. The sonographic appearances in postpartum thyroiditis. Clin Radiol 1992;45:311-5. [Crossref] [PubMed]
- Creagh FM, Parkes AB, Lee A, et al. The iodide perchlorate discharge test in women with previous post-partum thyroiditis: relationship to sonographic appearance and thyroid function. Clin Endocrinol (Oxf) 1994;40:765-8. [Crossref] [PubMed]
- Tachi J, Amino N, Tamaki H, et al. Long term follow-up and HLA association in patients with postpartum hypothyroidism. J Clin Endocrinol Metab 1988;66:480-4. [Crossref] [PubMed]
- Azizi F. The occurrence of permanent thyroid failure in patients with subclinical postpartum thyroiditis. Eur J Endocrinol 2005;153:367-71. [Crossref] [PubMed]
- Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011;21:1081-125. [Crossref] [PubMed]
- Azizi F, Braverman L. Management of postpartum thyrotoxicosis. Curr Opin Endocrinol Diabetes 2005;12:471-76. [Crossref]
- Azizi F. Treatment of post-partum thyrotoxicosis. J Endocrinol Invest 2006;29:244-7. [Crossref] [PubMed]
- Mestman J. Thyroid and parathyroid diseases in pregnancy. Obstetrics: normal and problem pregnancies. 5th edition. London: Churchill Livingstone, 2007:1023-9.
- Greene MF, Creasy RK, Resnik R, et al. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice E-Book. Elsevier Health Sciences, 2008.
- Goldstein AL. New-onset Graves' disease in the postpartum period. J Midwifery Womens Health 2013;58:211-4. [Crossref] [PubMed]
- de los Santos ET, Starich GH, Mazzaferri EL. Sensitivity, specificity, and cost-effectiveness of the sensitive thyrotropin assay in the diagnosis of thyroid disease in ambulatory patients. Arch Intern Med 1989;149:526-32. [Crossref] [PubMed]
- Amino N, Tanizawa O, Mori H, et al. Aggravation of thyrotoxicosis in early pregnancy and after delivery in Graves' disease. J Clin Endocrinol Metab 1982;55:108-12. [Crossref] [PubMed]
- Gonzalez-Jimenez A, Fernandez-Soto ML, Escobar-Jimenez F, et al. Thyroid function parameters and TSH-receptor antibodies in healthy subjects and Graves' disease patients: a sequential study before, during and after pregnancy. Thyroidology 1993;5:13-20. [PubMed]
- Yoshida S, Takamatsu J, Kuma K, et al. Thyroid-stimulating antibodies and thyroid stimulation-blocking antibodies during the pregnancy and postpartum period: a case report. Thyroid 1992;2:27-30. [Crossref] [PubMed]
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med 2003;348:2646-55. [Crossref] [PubMed]
- Momotani N, Noh J, Ishikawa N, et al. Relationship between silent thyroiditis and recurrent Graves' disease in the postpartum period. J Clin Endocrinol Metab 1994;79:285-9. [PubMed]
- Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am 1998;27:169-85. [Crossref] [PubMed]
- Tozzoli R, Bagnasco M, Giavarina D, et al. TSH receptor autoantibody immunoassay in patients with Graves' disease: improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun Rev 2012;12:107-13. [Crossref] [PubMed]
- Ide A, Amino N, Kang S, et al. Differentiation of postpartum Graves' thyrotoxicosis from postpartum destructive thyrotoxicosis using antithyrotropin receptor antibodies and thyroid blood flow. Thyroid 2014;24:1027-31. [Crossref] [PubMed]
- Ota H, Amino N, Morita S, et al. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves' disease. Clin Endocrinol (Oxf) 2007;67:41-5. [Crossref] [PubMed]
- Emerson C. Sporadic silent thyroiditis, postpartum thyroiditis, and subacute thyroiditis. Werner & Ingbar's the Thyroid, 2001.
- Arem R, Munipalli B. Ipodate therapy in patients with severe destruction-induced thyrotoxicosis. Arch Intern Med 1996;156:1752-7. [Crossref] [PubMed]
- Mountford PJ, Coakley AJ. A review of the secretion of radioactivity in human breast milk: data, quantitative analysis and recommendations. Nucl Med Commun 1989;10:15-27. [Crossref] [PubMed]
- Azizi F, Khoshniat M, Bahrainian M, et al. Thyroid function and intellectual development of infants nursed by mothers taking methimazole. J Clin Endocrinol Metab 2000;85:3233-8. [Crossref] [PubMed]
- Azizi F, Hedayati M. Thyroid function in breast-fed infants whose mothers take high doses of methimazole. J Endocrinol Invest 2002;25:493-6. [Crossref] [PubMed]
- Azizi F. Effect of methimazole treatment of maternal thyrotoxicosis on thyroid function in breast-feeding infants. J Pediatr 1996;128:855-8. [Crossref] [PubMed]
- Azizi F, Bahrainian M, Khamseh ME, et al. Intellectual development and thyroid function in children who were breast-fed by thyrotoxic mothers taking methimazole. J Pediatr Endocrinol Metab 2003;16:1239-43. [Crossref] [PubMed]
Cite this article as: Azizi F. Postpartum thyroid disorders. Ann Thyroid 2018;3:13.