
European perspective on active surveillance for papillary thyroid microcarcinoma—are we ready?
Introduction
Over the last decade much has changed in the diagnostic and therapeutic approaches to patients with differentiated thyroid cancers, and particularly to patients with papillary thyroid carcinoma (PTC). The change was noted in respect to the recommended extent of surgical treatment and to the indications for adjuvant radioiodine therapy in a low-risk patient group (1,2).
According to the 2009 guidelines of the American Thyroid Association (ATA), lobectomy should be performed in patients with papillary thyroid microcarcinoma (PTMC) (unifocal cancer ≤1 cm in diameter) with no lymph node involvement (3).
In 2015, indications for lobectomy were extended to cT2N0M0 (intrathyroidal tumor ≤4 cm in diameter) (1). At that time, indications for prophylactic central lymph node dissection were practically limited to T3–T4 cancers (1). In addition, in 2015, ATA recommendations considered active surveillance (AS) a possible management option in a selected group of patients with low-risk PTC (1), which initiated a broader and novel new therapeutic approach to this group of patients outside of Japan.
The ATA guidelines have a significant influence on the recommendations on the diagnosis and treatment of patients with thyroid cancer in other countries, including those in Europe where tendency to treatment de-escalation in patients with PTC is also observed.
However, a change in the therapeutic approach is not an easy process. In some countries, e.g., in Poland, abandonment of radical management in PTMC met with considerable resistance in the case of patients and the medical community. After several years of fierce debate (4,5), lobectomy in patients with PTMC (cT1aN0M0) finally became a possible, common and recommended treatment modality as late as in 2015 (2). Since that time, it has been performed successfully.
A very good or even excellent prognosis in PTMC has been observed and reported for many years (2,6). Some PTMCs are not clinically significant, which is confirmed by post-mortem studies. Frequent and incidental findings of PTMC in patients who died due to other causes [in over 30%, e.g., in the Finnish study (7,8)] indicated that this type of cancer was not characterized by an aggressive growth, nor did it affect the clinical condition of those patients. The insignificant percentage of small, clinically silent cancers detected incidentally after surgery for nodular goiter (from 3.6–10%) also confirms the low malignancy of PTMC (9,10).
On the other hand, the development of diagnostic techniques, the wide availability of modern ultrasound devices and the use of fine needle aspiration biopsy (FNAB) have had an influence on the increased worldwide incidence and diagnosis of low-risk PTC, particularly in high-income countries (6). As a result, thyroid cancer has not been a rare malignancy as it was observed in the 1980s and the 1990s. Therefore, both physicians and patients are forced to face the “worldwide thyroid cancer epidemic” (11).
The increase in the incidence of PTC has not resulted in an increase in mortality rates, which suggests overdiagnosis that could lead to overtreatment (6,11). More than 50% of PTCs have a diameter <1 cm (12), which again opened the discussion on the optimal therapeutic management in patients with an excellent clinical course and prognosis.
In addition, recently more attention has been paid to the need for personalized treatment, reduction of complications and adverse events, improvement in the quality of life of patients and also to the need for considering the benefit-risk ratio resulting from diagnostic and therapeutic management (1,2). The above factors contributed to the development of a strategy of AS in patients with low-risk PTC as opposed to immediate surgical intervention even of limited extent (lobectomy).
Active surveillance as a novel therapeutic option
Active surveillance is currently a novel option in the therapeutic algorithm for PTC. The first reports on the possibility for safe use of this strategy came from Japan (13-17). Currently, there are robust data from the Far East, i.e., Japan and Korea (18-20) and the United States (21,22), which show excellent oncologic outcomes in patients undergoing AS for PTC (6).
Based on the above reports, Brito et al. (23) developed the proposal of the algorithm for the enrollment in AS based on clinical patient characteristics, tumor/neck ultrasound characteristics and the medical team characteristics (Table 1) to standardize the indications for this treatment modality and to make it more common.
Table 1
| AS candidate | Approach |
|---|---|
| AS ideal candidate | |
| Patient features | Older patients (>60 years) |
| Acceptance of AS approach and future surgery if needed | |
| Reliable and compliant with follow-up | |
| Significant comorbid conditions or limited life expectancy | |
| US characteristics | Solitary thyroid nodule with well-defined margins |
| Nodule surrounded by >2 mm of normal gland parenchyma | |
| No evidence of ETE | |
| Imaging documenting stable size of nodule over time | |
| No evidence of nodal metastases | |
| No evidence of distant metastases | |
| Others tumor features | No high-risk features on cytological or molecular studies |
| Medical team features | Experienced multidisciplinary management team |
| High-quality neck ultrasonography available | |
| Prospective data collection | |
| Tracking/reminder program to ensure proper follow-up | |
| AS appropriate candidate | |
| Patient features | Younger patients (18–59 years) |
| Acceptance of AS approach and future surgery if needed | |
| Reliable and compliant with follow-up | |
| Strong family history of papillary thyroid cancer | |
| Child bearing potential | |
| US characteristics | Multifocal PTMC |
| Nodule with subcapsular location away from critical structures (i.e., RLN) | |
| No evidence of ETE | |
| Ill-defined margins | |
| Background US findings making follow-up assessment difficult (multiple benign, thyroid nodules, thyroiditis, nonspecific enlarged LNs) | |
| Others tumor features | FDG avid PTMC |
| Medical team features | Experienced endocrinologist or thyroid surgeon |
| Neck ultrasonography routinely available | |
| AS inappropriate candidate | |
| Patient features | Young patients (<18 years) |
| Non-compliance with the follow-up | |
| Non-acceptance of surgical approach | |
| US characteristics | Subcapsular location near RLN |
| Evidence of ETE | |
| Evidence of invasion into the trachea or esophagus | |
| Clinically evident nodal metastases | |
| Clinically evident distant metastases | |
| Increase in size on imaging | |
| Others tumor features | Aggressive features on cytological studies |
| Medical team features | Reliable neck ultrasonography not available |
| Little experience with thyroid cancer management |
AS, active surveillance; ETE, extrathyroidal extension; LNs, lymph nodes; PTMC, papillary thyroid microcarcinoma; RLN, recurrent laryngeal nerve US, ultrasound.
Even in Japan which pioneered this novel management strategy at Kuma Hospital in 1993, the indications and recommendations for AS vary significantly between institutions. Hence, to improve further implementation of this management modality, physicians and patients (even in Japan) should be further educated, and the sociomedical environment needs to be improved (18).
Of note, however, the research on AS has only come from a few worldwide centers. Of all European countries, AS has been conducted in only one center in Italy as part of a prospective non-randomized observational study with a relatively short medial follow-up of 19 months (24). The results of the Italian study are in line with the Japanese outcomes.
Disease progression and delayed surgery were observed in a similar percentage of cases, which did not influence the treatment results. The study confirmed the possibility of safe AS in a selected group of patients with the cytological diagnosis of PTC or suspected PTC (Bethesda V and VI) (25). Interestingly, of all the eligible patients, 50% of subjects (93/185) initially gave consent for enrollment in the proposed observational management and another 20% of patients (19/93) decided to transition to surgical intervention even though there was no evidence of disease progression. Therefore, the follow-up was continued in 74 patients as planned, i.e., in 40% of patients who were initially recommended such management. Additionally, 3 patients showed clinical progression and required surgical intervention. Currently, 71 of enrolled patients are still on AS (24). The study is conducted in a tertiary referral center with extensive experience in the diagnosis and treatment of thyroid cancer. Therefore, this method cannot yet be commonly used, which was emphasized by the authors of the study (24).
However, there has also been a recent increase in interest in this therapeutic option in patients with low-risk PTC also in other European countries. A Danish team conducted a retrospective study on a group of 803 patients. Their aims of the analysis were to assess the incidence and the outcome of PTMC depending on the method of tumor detection and to identify patients that may be suitable for AS. The authors stressed that PTMC patients without suspicion of metastasis had the same low risk of recurrence as incidental cases and could be candidates for AS (25).
Are we ready for active surveillance in Europe?
Considering the possibility of introducing AS into the therapeutic management of PTMC in Europe, several important aspects should be taken into account. Firstly, in the European population, multinodular goiter is more prevalent compared to a single lesion in the thyroid gland, which makes it difficult to follow up the lesion. In addition, a significant percentage of autoimmune thyroiditis is currently reported in Europe. In these cases, a characteristic ultrasound image may also hinder AS (2).
It seems that European patients and their attending physicians are characterized by considerably greater fear of the consequences of cancer disease and by aiming at a rapid and radical solution to the problem.
This attitude was observed when a Polish prospective clinical trial on thyroid cancer staged T1N0M0 was initiated. The aims of study were to make lobectomy more commonly applied in Poland and to reduce prophylactic central lymph node dissection in low-risk PTC (4,5). The implementation of lobectomy as a therapeutic option in PTMC required many years of effort to convince the medical community, particularly endocrinologists (2).
Active surveillance requires an experienced medical team and patients’ complete acceptance of such management, strong motivation and determination, which was stressed by the Italian team (24).
Ito et al. paid attention to the problem of implementation of AS (26). The number of patients whose therapeutic strategies were determined by endocrinologists increased compared to the situation when the treatment method was determined by surgeons (26). Therefore, next to motivation of patients, the treatment team must also be convinced about the relevance of such management.
Currently, it seems that in European countries, including Poland, the community of endocrinologists is not yet ready for the introduction of AS. Endocrinologists working in oncology centers are more likely to implement this strategy as they are more experienced in the treatment and management of thyroid cancer patients.
In addition, there is no clear evidence of cost reduction of such management for the healthcare system in different countries. On the one hand, the Japanese study showed more than a 4-fold decrease in costs of AS compared to immediate surgical intervention in a 10-year follow-up (27). On the other hand, the Australian cohort showed that surgery may have a long-term economic advantage for younger Australian patients with PTMC who are likely to require more than 16 years of follow-up in an AS surveillance scheme (28). Therefore, in young patients at higher risk of recurrence, immediate surgery seems to be more beneficial from the economic perspective (28).
Standardized recommendations for AS have not been established yet. A uniform schedule of follow-ups for patients undergoing AS has not been developed either. The psychological aspect of life in cancer patients cannot be neglected when the possibility of the implementation of AS is considered on a larger scale.
Therefore, it is important to assess their quality of life during AS, as well as to evaluate and compare the quality of life of patients after immediate surgery (29-31). Such initial reports have already been published (29-32).
Elderly patients with comorbidities seem to be better candidates for AS (23). However, if progression is observed in this group of patients, postponement of surgery may be associated with a higher perioperative risk due to comorbidities and general anesthesia.
It is also confirmed that cancer progression or dissemination is also found in some patients despite the generally good prognosis of patients with PTC and mostly excellent prognosis for PTMC (33,34).
Currently, it seems crucial to identify patients at high risk of tumor progression in whom AS could practically contribute to worsening the prognosis. Therefore, studies should be continued on the prognostic and predictive molecular classifiers, especially those that could assess the molecular profile of the tumor based on preoperative FNAB.
Such molecular classifiers have not been widely available in Europe yet. They are currently at the clinical trial stage (35). In the USA where molecular tests are the most commonly used, they are not standard in everyday clinical practice (1).
In 2019, Xing published the paper on prognostic genetic marker-guided risk stratification and management of thyroid cancer in which the author proposed the algorithm for therapeutic management depending on the type of tumor mutation. Active surveillance seems to be reasonable only for clinically low-risk wild-type BRAF PTMC (Figure 1) (34).
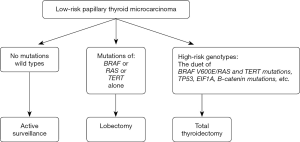
However, other authors questioned the role of the BRAF mutation as a single, independent factor on which therapeutic decisions related to PTC should be based. Although its significance for a poor prognosis has been confirmed (34,36,37), the mutation was found in more than 50% of PTMCs (38,39). Also, most of them are still characterized by excellent prognosis and an indolent clinical course. The role of the TERT mutation for the unfavorable clinical course of PTMCs is not clearly confirmed either (34,40). It seems that at the time of enrollment of patients with PTMCs for AS, the co-occurrence of various mutations associated with tumor progression should be considered, e.g., BRAF, TERT, p53, CTNNB1 (34,41).
Finding sensitive and specific molecular markers which allow a better assessment of possible disease progression may contribute to the use of AS on a larger scale as a management option for patients with thyroid cancer. However, further research is warranted and currently it is a matter of the future. Nevertheless, as the example of prostate cancer shows, this is the right direction of further research as indicated by Klein et al. using a robust and validated prognostic signature (Decipher®). Therefore, molecular profiling of such tumors may better select patients for AS at diagnosis and trigger appropriate intervention during follow-up (42).
Ze et al. searched the available databases (until May 2019) and observed that only 4 centers worldwide recruited patients in their registered AS studies. Those trials included Canada, Hong Kong, South Korea and the United States (33). However, no such trial is currently conducted in Europe. Perhaps it would be possible to plan a multicenter European trial based on a uniform protocol. Patients with low-risk PTC account for over 40% of diagnosed PTC, which is a significant population that requires modern and individualized therapeutic management (43-47). In the future, such management could be introduced to the centers other than tertiary referral hospitals, which could be beneficial for patients with a favorable prognosis.
Summary
Currently, AS can be one of the management options for a selected group of patients with PTC. The choice of the therapeutic modality should be based on the preferences of patients and treatment teams as well as on an objective assessment of risk factors for tumor progression, which is currently neither obvious nor conclusive.
It seems that in Europe, this strategy as a management option for low-risk PTC should be considered. Bearing in mind legal aspects, it should be currently limited to clinical trials and conducted only in experienced centers. For this moment, however, we are not ready for the common use of AS in clinical practice in Europe.
Acknowledgments
We acknowledge the linguistic assistance provided by Assistant Professor Arkadiusz Badziński, PhD, a medical translator and interpreter, in the preparation of this manuscript.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Thyroid for series “Recent Challenges in the Management of Thyroid Tumors”. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/aot-20-37
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-20-37). The series “Recent Challenges in the Management of Thyroid Tumors” was commissioned by the editorial office without any funding or sponsorship. Marcin Barczynski served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Thyroid from Oct 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Jarząb B, Dedecjus M, Handkiewicz-Junak D, et al. Diagnostics and Treatment of Thyroid Carcinoma. Endokrynol Pol 2016;67:74-107. [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [Crossref] [PubMed]
- Czarniecka A, Półtorak S, Sacher A, et al. Surgical strategy in low advanced differentiated thyroid cancer staged T1N0M0- results of a pilot feasibility study. Eur Thyroid J 2014;3:104.
- Czarniecka A, Półtorak S, Sacher A, et al. The assessment of treatment of patients with low advanced papillary thyroid cancer staged cT1N0M0 treated as part of prospective clinical trial. Endokrynol Pol 2015;A34.
- Lohia S, Hanson M, Tuttle RM, et al. Active surveillance for patients with very low-risk thyroid cancer. Laryngoscope Investigative Otolaryngology 2020;5:175-82. [Crossref] [PubMed]
- Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A "normal" finding in Finland. A systematic autopsy study. Cancer 1985;56:531-8. [Crossref] [PubMed]
- Lang W, Borrusch H, Bauer L. Occult carcinomas of the thyroid. Evaluation of 1,020 sequential autopsies. Am J Clin Pathol 1988;90:72-6. [Crossref] [PubMed]
- Pelizzo MR, Piotto A, Rubello D, et al. High prevalence of occult papillary thyroid carcinoma in a surgical series for benign thyroid disease. Tumori 1990;76:255-7. [Crossref] [PubMed]
- Takebe K, Arai T. Problems of thyroid ultrasound screening-based on the results of thyroid screening conducted at our facility 25 years ago in Japan. Ultrasound Med Biol 2017;43:S114. [Crossref]
- Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 2016;375:614-7. [Crossref] [PubMed]
- Morris LG, Sikora AG, Tosteson TD, et al. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid 2013;23:885-91. [Crossref] [PubMed]
- Sugitani I, Toda K, Yamada K, et al. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes World J Surg 2010;34:1222-31. [Crossref] [PubMed]
- Ito Y, Uruno T, Nakano K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003;13:381-7. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Inoue H, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 2010;34:28-35. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kihara M, et al. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid 2014;24:27-34. [Crossref] [PubMed]
- Miyauchi A, Kudo T, Ito Y, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery 2018;163:48-52. [Crossref] [PubMed]
- Sugitani I, Ito Y, Miyauchi A, et al. Active surveillance versus immediate surgery: questionnaire survey on the current treatment strategy for adult patients with low-risk papillary thyroid microcarcinoma in Japan. Thyroid 2019;29:1563-71. [Crossref] [PubMed]
- Kwon H, Oh HS, Kim M, et al. Active surveillance for patients with papillary thyroid microcarcinoma: a single center’s experience in Korea. J Clin Endocrinol Metab 2017;102:1917-25. [Crossref] [PubMed]
- Kim TY, Shong YK. Active surveillance of papillary thyroid microcarcinoma: a mini-review from Korea. Review Endocrinol Metab (Seoul) 2017;32:399-406. [Crossref] [PubMed]
- Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg 2017;143:1015-20. [Crossref] [PubMed]
- Tuttle RM, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab 2018;13:77-85. [Crossref] [PubMed]
- Brito JP, Ito Y, Miyauchi A, et al. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid 2016;26:144-9. [Crossref] [PubMed]
- Molinaro E, Campopiano MC, Pieruzzi L. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at one single Italian center. J Clin Endocrinol Metab 2020;105:dgz113. [PubMed]
- Reinke R, Mathiesen JS, Larsen SR, et al. A study from The Danish Thyroid Cancer Group - DATHYRCA (part of the DAHANCA organization). Incidental and non-incidental papillary thyroid microcarcinoma in Denmark 1996-2015: A national study on incidence, outcome and thoughts on active surveillance. Cancer Epidemiol 2019;60:46-50. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. Trends in the implementation of active surveillance for low-risk papillary thyroid microcarcinomas at Kuma Hospital: gradual increase and heterogeneity in the acceptance of this new management option. Thyroid 2018;28:488-95. [Crossref] [PubMed]
- Oda H, Miyauchi A, Ito Y, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J 2017;64:59-64. [Crossref] [PubMed]
- Lin JF, Jonker PKC, Cunich M, et al. Surgery alone for papillary thyroid microcarcinoma is less costly and more effective than long term active surveillance. Surgery 2020;167:110-6. [Crossref] [PubMed]
- Jeon MJ, Kim WG, Chung KW, et al. Active surveillance of papillary microcarcinoma: Where do we stand? Eur Thyroid J 2019;8:298-306. [Crossref] [PubMed]
- Jeon MJ, Lee YM, Sung TY, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: A cross-sectional study. Thyroid 2019;29:956-62. [Crossref] [PubMed]
- Kong SH, Ryu J, Kim MJ, et al. Longitudinal assessment of quality of life according to treatment options in low-risk papillary thyroid microcarcinoma patients: Active surveillance or immediate surgery (Interim analysis of MAeSTro) Thyroid 2019;29:1089-96. [Crossref] [PubMed]
- Yoshida Y, Horiuchi K, Okamoto T. Patients' view on the management of papillary thyroid microcarcinoma: active surveillance or surgery. Thyroid 2020;30:681-7. [Crossref] [PubMed]
- Ze Y, Zhang X, Shao F, et al. Active surveillance of low-risk papillary thyroid carcinoma: a promising strategy requiring additional evidence. J Cancer Res Clin Oncol 2019;145:2751-9. [Crossref] [PubMed]
- Xing M. Prognostic genetic marker-guided risk stratification and management of thyroid cancer. Endocrinol Metab Clin North Am 2019;48:109-24. [Crossref] [PubMed]
- Oczko-Wojciechowska M, Kotecka-Blicharz A, Krajewska J, et al. European perspective on the use of molecular tests in the diagnosis and therapy of thyroid neoplasms. Gland Surg 2020;9:S69-76. [Crossref] [PubMed]
- Li F, Chen G, Sheng C, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: A meta-analysis. Endocr Relat Cancer 2015;22:159-68. [Crossref] [PubMed]
- Chen Y, Sadow PM, Suh H, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: A systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid 2016;26:248-55. [Crossref] [PubMed]
- Yu FX, Hu MX, Zhao HX, et al. Precise detection of gene mutations in fine-needle aspiration specimens of the papillary thyroid microcarcinoma using next-generation sequencing. Int J Endocrinol 2019; [Crossref] [PubMed]
- Yabuta T, Matsuse M, Hirokawa M, et al. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid 2017;27:1206-7. [Crossref] [PubMed]
- de Biase D, Gandolfi G, Ragazzi M, et al. TERT promoter mutations in papillary thyroid microcarcinomas. Thyroid 2015;25:1013-9. [Crossref] [PubMed]
- Lantsov D, Meirmanov S, Nakashima M, et al. Cyclin D1 overexpression in thyroid papillary microcarcinoma: Its association with tumour size and aberrant beta-catenin expression. Histopathology 2005;47:248-56. [Crossref] [PubMed]
- Klein EA, Santiago-Jiménez M, Yousefi K, et al. Molecular Analysis of Low Grade Prostate Cancer Using a Genomic Classifier of Metastatic Potential. J Urol. 2017;197:122-8. [Crossref] [PubMed]
- Matrone A, Campopiano MC, Nervo A, et al. Differentiated thyroid cancer, from active surveillance to advanced therapy: toward a personalized medicine Front Endocrinol (Lausanne) 2020;10:884. [Crossref] [PubMed]
- Ramundo V, Sponziello M, Falcone R, et al. Low-risk papillary thyroid microcarcinoma: Optimal management toward a more conservative approach. J Surg Oncol 2020;121:958-63. [Crossref] [PubMed]
- Saravana-Bawan B, Bajwa A, Paterson J, et al. Active surveillance of low-risk papillary thyroid cancer: A meta-analysis. Surgery 2020;167:46-55. [Crossref] [PubMed]
- Cho SJ, Suh CH, Baek JH, et al. Active surveillance for small papillary thyroid cancer: A systemic review and meta-analysis. Thyroid 2019;29:1399-408. [Crossref] [PubMed]
- Sugitani I. Active surveillance for very low-risk papillary thyroid carcinoma: experience and perspectives from Japan. Ann Thyroid 2018;3:26. [Crossref]
Cite this article as: Czarniecka A, Oczko-Wojciechowska M, Barczynski M. European perspective on active surveillance for papillary thyroid microcarcinoma—are we ready? Ann Thyroid 2020;5:15.


