
Autofluorescence imaging: a tool for intraoperative mapping of the parathyroid gland
Introduction
The primary aim of surgery in patients with well-differentiated thyroid cancer is complete removal of the tumor. However, considering the excellent survival rate, preventing complications is as important as removal of the tumor because post-thyroidectomy complications such as vocal cord paralysis or permanent hypocalcemia can be very painful to both surgeons and patients. Thus, quality of life after surgery is a critical issue for thyroid cancer patients. In this regard, central tenant of thyroidectomy has been to minimize complication. As for the preservation of the recurrent laryngeal nerve, intraoperative nerve monitoring using endotracheal electromyography tube has been widely accepted. Surgeons now can easily map, identify and monitor the nerve function during thyroid and parathyroid surgery (1).
However, regarding the identification and preservation of normal parathyroid gland (PGs), there has been no reliable intraoperative method. Thus, the solution has been mostly based on intuitive visual inspection which can be gained through extensive surgeon experiences (2). Above all, early identification of the PGs during surgery is essential for preservation of the PGs. This is challenging because of their small size, various location, and ambiguous shape as well (3,4). Furthermore, precise identification and preservation of PGs can be difficult even for highly skilled surgeons under some situations, such as distorted anatomy from previous surgery, intrathyroidal glands, presence of ectopic or super-numerous glands, extensive tumor invasion (4).
According to the International Thyroid Cancer Patient Survey (5), hypocalcemia is the most common complication after thyroidectomy and also the most common unresolved problem persisting more than 1 year since diagnosis. It may accompany perioral numbness, paresthesia of hand or foot, muscle cramp, Trousseau sign, Chvostek’s sign, laryngeal spasm, seizure, anxiety, confusion, QT interval prolongation, and even cardiac arrest. To avoid this complication, several techniques such as frozen biopsy, fine needle aspiration (FNA) and parathyroid hormone (PTH) assay, methylene blue staining or indocyanine green (ICG) injection, have been introduced. But they showed limited roles in localizing PGs during thyroidectomy (6-9). Our interests to minimize post-thyroidectomy hypoparathyroidism led us to study near infrared autofluorescence (NIR AF) imaging of PGs.
Recently, AF imaging with near infra-red (NIR) light for parathyroid identification has attracted attentions. In 2011, Paras et al. (10) reported a landmark study presenting variable range of AF intensity in neck organs. They demonstrated that PGs had the strongest intensity at 820 nm followed by very weak intensity of the thyroid gland and no AF in surrounding fat, muscle, and trachea. Additional studies on imaging of parathyroid AF using a commercially available near-infrared camera, Fluobeam® (Fluoptics, Grenoble, France) have been reported to confirm the presence of parathyroid tissue in the surgical field.
We built a NIR AF imaging device at the laboratory level and used it to introduce imaging technology to show the presence and spatial information of AF in real time (11). More recently, we have reported the concept of ‘parathyroid gland mapping’ defined as definitive identification of PGs through localization process for PGs which was initially not visualized by naked eye (12). It is thought that this early localization of the PGs may minimize manipulation of the PGs and prevent inadvertent injury to vessels. This may reduce the incidence of post-thyroidectomy hypocalcemia. We review current papers that describe AF imaging of PGs during thyroid and parathyroid surgery are reviewed.
AF of PGs
AF, or native fluorescence, is a phenomenon that consists of the emission of fluorescence light in a slightly longer spectral range when the biological substrates are excited with light at suitable wavelengths. Since there is an exact relationship between-endogenous fluorophores and morpho-functional properties of biological system, this can provide accurate diagnostic information without removing the tissue specimen (in situ). As AF imaging does not require any exogenous dye, it has the advantage over non-invasive techniques (13).
Paras et al. (10) showed that PG has fluorescence properties at NIR wavelengths using spectroscopy. They showed that the PGs and thyroid glands emit light at a wavelength of 820 nm when illuminated with light around 785 nm wavelength respectively (Figure 1). The authors demonstrated that the PGs had the strongest AF intensity regardless of healthy or diseased states. On the other hand, the thyroid gland had lower intensity, and other structures (including trachea, lymph node, muscle, etc.) had no significant AF (7). The NIR endogenous fluorophore in PGs have not yet been definitively identified, but suggested candidates include a co-localized protein in the secretory granule or calcium-sensing receptors or vitamin D receptors in the cell wall (7,14,15).
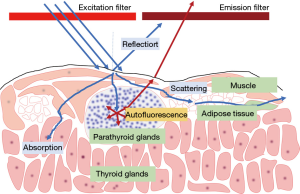
So far, studies on NIR wavelengths that are attractive in biomedicine and clinical applications have mainly been performed by administrating ICG. ICG is an exogenous contrast, which has been widely used as a non-specific agent to detect sentinel lymph nodes during surgery. In parathyroid surgery, ICG has been applied for the localization of diseased PG (8,16). For the evaluation of parathyroid blood flow, intravenous ICG angiography can be conducted. This method helps to predict the function of the PGs during surgery. However, it is not used as a screening tool to find the PGs intraoperatively (17,18).
Intraoperative identification of PG s with AF
Parathyroid tissue is not easily distinguished by the naked eye. In addition, the blood vessels that supply the PGs are very fine and the vascular distribution is difficult to predict. Thus, the operator must proceed very carefully to dissect the tissue at the site where the PGs are expected to be present (19). However, the incidence of inadvertent PG removal has been reported to vary from 9.1–15%. Additionally, autotransplantation has been performed at 15–25% (20).
As for the application potential of AF in neck surgery, promising advancements pertain to the intraoperative localization of the PGs. PGs show much stronger fluorescence intensity than other neck structures even without exogenous contrast agent. This optical property was investigated by analyzing the captured images. The PGs detected by the operator’s naked eye during surgery was compared with the image. As AF signal of PGs is very weak, a modified NIR fluorescence imaging system (Karl Storz, PDD camera) was used to detect this signal (21).
Since that time, many researchers have begun to focus on the possibility of fluorescent image-guided thyroid or parathyroid surgery to identify PGs in real-time. With the advances in light source for excitation, detection devices (including fiber optic probes for excitation and AF light-guides, and a fine-tuned camera for detection of AF emission) and data analysis procedures, many studies are being actively conducted to optically identify the parathyroid tissue.
Commercially available systems for AF-based parathyroid identification
Currently, 2 commercialized devices including Fluobeam® (Fluoptics, Grenoble, France) as a NIR AF imaging system and PTeye® (AiBiomed Inc, Santa Barbara, CA, and Vanderbilt University, Nashville, TN) as a NIR AF fiber probe-based system, both of which were approved by the FDA in 2018. The Fluobeam® is contact-free and visualizes all structures adjacent to the PGs in the surgical field. This imaging device could be valuable for the surgeon to intuitively understand the spatial relationship of the surgical site structures. Fluobeam® can capture a fluorescence image during operations. In addition, it does not require contact with the surgical field or change of the operative workflow. However, there are still some problems to be technically solved in terms of user interface to check the image in real surgical situations. Capturing the image using a medical device can be obtained only at a working distance of 10–30 cm from the operative field (22). Also, the device is hand guided and requires maneuvering the device while observing the AF image on the remote monitor until the best image is captured. Since NIR AF of the PGs is a considerably weak intensity light, it is necessary to turn the room lights off to make the operating room completely dark when capturing the AF image. This may disrupt the surgical process and extend operative times.
PTeye® is compact, easy to transport and store, and ensures effectiveness even in the presence of ambient lights such as operating light, room light and head light. This probe type device is used in contact with tissue and check the PGs with point-by-point measurements. PTeye® provides real-time quantitative information. However, it does not show spatial information of the operation site, and may also be more disruptive to the surgical flow.
Other systems for intraoperative identification of PG s with AF
The limitation of the NIR imaging device is that the surgical lighting obscures the AF. Thus, the signal-to-background ratio is decreased and AF is not represented in the image when the operating light is on. Furthermore, since the intensity of the AF is considerably weak, it is necessary to maintain a dark room with all indoor lights off to properly capture the images. This issue can be usually solved by turning off the operating light during AF detection and applying a specific emission filter that blocks the wavelength of fluorescence room light.
To overcome some limitations of the above commercialized equipment, we developed lab built Near-Infrared AF Imaging system using DSLR camera and NIR AF probe (Figure 2). Our NIR camera system developed in the laboratory, consists of a digital single lens reflex camera, an excitation light, and two narrow bandpass filters for excitation and emission. Since the image sensor of our system is used, high-quality images can be obtained (11,12). With the NIR camera we have modified, the operator can produce seamless live video-imaging on the surgical field. Images of surrounding tissues can be acquired regardless the depth of field, even with the room light on. Also, it has a working distance of approximately 80–100 cm, which is fixed over the operator’s head during surgery. Thus, a two-handed operation can be performed in a convenient setting without repeated disruption of the operative procedure. By viewing AF live images from the surgical field on a remote display monitor, the surgeon can correlate the hand movement and instrument manipulation in real time, so that the surgical work flow is not interrupted (Figure 3).
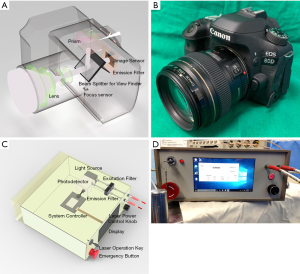

We made another camera system detecting the AF signal of PGs. Main components of this system are the Raspberry Pi development board and camera. A specially designed emission filter was assembled, which allows the passage of 1/100 of visible wavelength and nearly all infrared light longer than 808 nm, so that a weak AF signal can be obtained even with active ambient room lighting. The surgical field was also represented by the visible light imaging reflected by the ambient room light. As there is a limit to the quality generated thus far, this device is in the process of improvement (23).
Recently, a preliminary report using a goggle-based system was introduced. Image acquisition system composed by a night vision goggle device with the bandpass filter was used instead of the NIR camera to detect the fluorescence signal (24). It has been suggested that this could facilitate the development of various devices that can be easily assembled and widely used at low cost.
‘PG mapping’ of unexposed PGs with NIR AF imaging
The NIR wavelength, compared to the ultra-violet (UV) or visible light wavelength, is optimal for biomedical applications. In medical physics, the optical window is the wavelength region wherein living tissue absorbs relatively scant light as part of the visible and infrared spectra, which is an area approximately 650–1,200 nm. At shorter wavelength, light is strongly absorbed by hemoglobin in the blood, while at longer wavelengths, water absorbs infrared light more strongly. For this reason, the NIR range with optical window 700–900 nm allows for deeper penetration depth of several millimeters with low scattering and low absorption (25). Using these optical properties, it became possible to set-up the parathyroid mapping technique to detect the AF of unexposed PGs before visual identification.
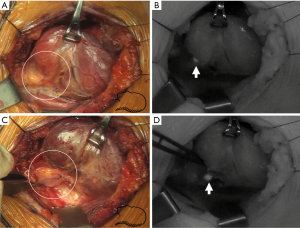
In the real clinical setting, blood vessels or connective tissue can block the excitation light and lead to false negative fluorescence images. However, in our previous study, the feasibility of the parathyroid mapping concept was presented, showing that PGs covered with fibrofatty tissues could be identified before the surgeon’s visual identification. The sensitivity and accuracy for PGs detection were 92.75% and 92.85%, respectively (12). Although it was a preliminary study, the results suggest that NIR AF of PGs below layers of fibrofatty tissue could be visualized (Figure 4).
Localizing deep-seated PGs under fatty tissue would eventually depend on the intensity of the AF signal, and it is obvious that tissues with higher fluorescence intensity are likely to be detected preferentially. In addition to PGs, the thyroid gland and brown adipose tissue also have AF properties. This is confirmed by conventional histology of tissues which are NIR AF positive (26). Some nodules of the thyroid gland have a higher signal than the normal thyroid fluorescence, including colloid nodules which may be characterized by a diffuse fluorescence signal. Bright spots that are similar to fluorescence may be observed in brown adipose tissue during NIR imaging. These bright spots were observed when NIR light was off as well or only when white light was illuminated. This means that the signal of brown adipose tissue may not be specific to NIR and these signals are not from PGs. The AF signal intensity of the PGs is 2–12 times higher than colloid nodules or brown adipose tissue and shows a clear fluorescence signal that is readily appreciated (11,12). This may be the reason why PGs could be identified even when the PGs are covered with fat or in the thyroid gland. More investigations are required to validate depth-based detection for the PGs.
Biological variables affecting AF signal
The clinician is interested in whether the patient's characteristics affect the fluorescence image. There have been studies that patient factors such as preoperative diagnosis, parathyroid hormone and serum calcium level, vitamin D level, body mass index (BMI) may influence the AF signal (7,27).
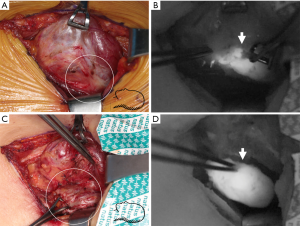
Studies on the influence of the pathology of PGs is ongoing. However, it is not known how the AF properties differ from normal functioning and the hyperfunctioning gland. Some preliminary work has reported that the hyperfunctioning glands have heterogenous and low-intensity AF compared with normal functioning glands. If the PGs show bright and/or homogeneous AF, they are less likely to be abnormal glands conversely. If low intensity and/or heterogeneous AF is detected, it suggests that the glands may be in a hyperfunctioning status. This concept could be implemented as another adjunctive parameter for predicting the pathologic status of the PGs (28) (Figure 5).
The PGs in renal-induced secondary hyperparathyroidism (r-SHP) have lower AF intensity than other diseased PGs. This fluorescence signal even may be lower than that of thyroid gland (27). This property may render NIR AF ineffective for the identification PGs for r-SHP. This could also raise concerns that it may be less effective in multi-gland disease processes. There are reports of significant negative correlations between AF and serum calcium. Serum PTH is an indicator of the degree of parathyroid function and reflects abnormality in the gland. However, the correlation with AF lacked statistical significance. No significant correlation was found between Sesta-MIBI positivity, gland weight, or gland composition. These variables may be surrogates for the degree of gland abnormality (27,29).
There is a learning curve for the correct interpretation of the AF images. Non-specific fluorescence may be observed brightly in a dye-processed blue drop or surgical threads. As above, a false-positive in the colloid nodule and brown fat may be bright. Additional increased intensity of signal can be generated when the illuminating light reflects off a metal instrument. These can cause confusion in interpreting the image. AF should be considered as an additional tool for an experienced surgeon rather than a replacement for appropriate expertise.
Effects of NIR-AF imaging on postoperative hypoparathyroidism
To prevent postoperative hypoparathyroidism, it is essential to identify the PGs during surgery and to ensure that the blood flow is not damaged, which is the indicator to predict if it will function well after surgery (30). However, there are no reliable non-invasive intraoperative methods that can predict and evaluate the postoperative function of the preserved PGs. The endogenous fluorophores in the parathyroid tissue related to AF emission have not yet been clearly characterized. Since AF is also expressed in PGs after it has already been extracted or contained in formalin, AF-based identification in situ does not guarantee postoperative parathyroid function. Some clinical studies have reported that NIR AF for total thyroidectomy could reduce postoperative hypocalcemia and the autotransplantation rate. Alternatively, a thyroid specimen that has been removed could be examined before being submitted to pathology using NIR AF. Missed PG s could then be harvested and autotransplanted.
In summary, intraoperative identification of PG s is a crucial step in preventing postoperative hypocalcemia in thyroid surgery. In our experience to date, PG mapping and localization is possible with a high rate using NIR AF imaging. We believe techniques of parathyroid AF will be increasingly used in thyroid and parathyroid surgery in the near future.
Acknowledgments
Funding: This was supported by National Research Foundation of Korea under grants (NRF-2017R1D1A1B03031082, NRF-2018R1A2B6005604, NRF-2019M3E5D1A 02070860).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathon Russell and Jeremy Richmon) for the series “The Management of Thyroid Tumors in 2021 and Beyond” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot-19-58). The series “The Management of Thyroid Tumors in 2021 and Beyond” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berri T, Houari R. Complications of thyroidectomy for large goiter. Pan Afr Med J 2013;16:138. [Crossref] [PubMed]
- Kim SW, Lee HS, Lee KD. Intraoperative real-time localization of parathyroid gland with near infrared fluorescence imaging. Gland Surg 2017;6:516-24. [Crossref] [PubMed]
- Lee NJ, Blakey JD, Bhuta S, et al. Unintentional parathyroidectomy during thyroidectomy. Laryngoscope 1999;109:1238-40. [Crossref] [PubMed]
- Lin DT, Patel SG, Shaha AR, et al. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope 2002;112:608-11. [Crossref] [PubMed]
- Thyroidcanceralliance. international-thyroid-cancer-survey 2010 [cited 2019 Sep. 30]. Available online: https://thyroidcanceralliance.org/
- Patel HP, Chadwick DR, Harrison BJ, et al. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg 2012;99:1345-51. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013;154:1371-7. [Crossref] [PubMed]
- Suh YJ, Choi JY, Chai YJ, et al. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc 2015;29:2811-7. [Crossref] [PubMed]
- Irvin GL 3rd, Molinari AS, Figueroa C, et al. Improved success rate in reoperative parathyroidectomy with intraoperative PTH assay. Ann Surg 1999;229:874. [Crossref] [PubMed]
- Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. [Crossref] [PubMed]
- Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab 2016;101:4646-52. [Crossref] [PubMed]
- Kim SW, Lee HS, Ahn YC, et al. Near-Infrared Autofluorescence Image-Guided Parathyroid Gland Mapping in Thyroidectomy. J Am Coll Surg 2018;226:165-72. [Crossref] [PubMed]
- Croce AC, Bottiroli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem 2014;58:2461. [PubMed]
- Thomas G, McWade MA, Sanders ME, et al. Identifying the novel endogenous near-infrared fluorophore within parathyroid and other endocrine tissues. Optical Society of America: Optical Tomography and Spectroscopy, 2016.
- Ladurner R, Lerchenberger M, Al Arabi N, et al. Parathyroid Autofluorescence—How Does It Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules 2019;24:2560. [Crossref] [PubMed]
- Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [Crossref] [PubMed]
- Kahramangil B, Berber E. The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 2017;115:848-55. [Crossref] [PubMed]
- Edafe O, Antakia R, Laskar N, et al. Systematic review and meta‐analysis of predictors of post‐thyroidectomy hypocalcaemia. Br J Surg 2014;101:307-20. [Crossref] [PubMed]
- Sasson AR, Pingpank JF Jr, Wetherington RW, et al. Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg 2001;127:304-308. [Crossref] [PubMed]
- McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014;99:4574-80. [Crossref] [PubMed]
- Zhu B, Sevick-Muraca E. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br J Radiol 2015;88:20140547. [Crossref] [PubMed]
- Kim Y, Kim SW, Lee KD, et al. Real-time localization of the parathyroid gland in surgical field using Raspberry Pi during thyroidectomy: a preliminary report. Biomed Opt Express 2018;9:3391-8. [Crossref] [PubMed]
- Serra C, Silveira L, Canudo A, et al. Parathyroid identification by autofluorescence–preliminary report on five cases of surgery for primary hyperparathyroidism. BMC Surg 2019;19:120. [Crossref] [PubMed]
- Tanaka E, Choi HS, Fujii H, et al. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann Surg Oncol 2006;13:1671-81. [Crossref] [PubMed]
- De Leeuw F, Breuskin I, Abbaci M, et al. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J Surg 2016;40:2131-8. [Crossref] [PubMed]
- McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016;159:193-202. [Crossref] [PubMed]
- Kose E, Kahramangil B, Aydin H, et al. Heterogeneous and low-intensity parathyroid autofluorescence: Patterns suggesting hyperfunction at parathyroid exploration. Surgery 2019;165:431-7. [Crossref] [PubMed]
- DiMarco A, Chotalia R, Bloxham R, et al. Autofluorescence in Parathyroidectomy: Signal Intensity Correlates with Serum Calcium and Parathyroid Hormone but Routine Clinical Use is Not Justified. World J Surg 2019;43:1532-7. [Crossref] [PubMed]
- Promberger R, Ott J, Kober F, et al. Intra-and postoperative parathyroid hormone-kinetics do not advocate for autotransplantation of discolored parathyroid glands during thyroidectomy. Thyroid 2010;20:1371-5. [Crossref] [PubMed]
Cite this article as: Kim SW, Lee HS, Kim Y, Lee KD. Autofluorescence imaging: a tool for intraoperative mapping of the parathyroid gland. Ann Thyroid 2020;5:12.


