
Thyroid function in individuals living with human immunodeficiency virus: the concern and debate about regular screening
Introduction
Human immunodeficiency virus (HIV) infection is one of the most severe diseases globally, with approximately 940,000 deaths in 2017 (1); and is known to cause progressive decrease in CD4 T cells, which leads to immune system failure, increase in vulnerability to opportunistic infections and cancer development. HIV prognosis has improved over the years, as a result of antiretroviral regimens.
Many disease conditions generated by HIV have been increasingly reported; such as abnormalities of pancreas, endocrine and gonadal metabolisms (2,3). In addition, studies have also shown antiretroviral medications induce complications such as lipoatrophy, hypercholesterolaemia and glucose intolerance (4).
In particular, there have been controversies on the influence of HIV on thyroid functioning. Reports on the increased incidence of thyroid disease amongst HIV patients compared to the general population have been published (5). The manifestations of thyroid problems reported include both functional disturbances and pathological changes. Although, the mechanism behind thyroid disease amongst HIV infected patients has been unclear, some possible explanations have been made; such as the opportunistic pathogens induce reversible thyroid organ destruction (6). Thyroid hormones are hugely essential for varied metabolic activities in the body, thus such dysfunction can decrease the quality of life of HIV patients; thereby changing the disease course. The evidence we will provide in this review will help to decide about the cost effectiveness of the importance of screening for thyroid function. Therefore, in this review we will aim to provide full review of the literature about the impact of HIV in the thyroid function and its consequence on patients. The findings of this review can assist in accentuating the necessity of thyroid abnormality screening for HIV infected patients, which could also contribute to treatment guidelines.
Hypothyroidism
Clinical manifestations of thyroid hormones deficiency are very diverse from patients being asymptomatic to presenting with critical illness. Often symptoms of hypothyroidism include fatigue, cold intolerance, depression and weight gain (7). Such symptoms are quite ambiguous and are usually not reported to a medical professional until late. A simple biochemical test illustrating elevated serum thyroid stimulating hormone (TSH) and reduced serum free thyroxine 4 (FT4) levels indicates diagnosis.
Beltran et al. [2003] research revealed that hypothyroidism was the most common thyroid dysfunction amongst 343 HIV patients. Hypothyroidism presented as overt, subclinical and reduced FT4 levels. They illustrated that the lower the CD4 T cells count of a patient the greater the risk of developing hypothyroidism (8). This was also illustrated by Collazos et al. [2003] (6). The ambiguous presentation of hypothyroidism can be easily attributed to symptoms related to HIV or HIV therapy or even related to opportunistic infections. Therefore, it’s important that clinician treating individuals LWHIV have broader understanding of different conditions and screen for thyroid function.
Overt hypothyroidism
Besides the obvious reduced metabolic rate that overt hypothyroidism induces, wide range of organs and systems are also implicated; which include, neuropathy, cochlear dysfunction, impairment of cognitive functioning, infertility, anaemia and dysregulation of glucose metabolism (7); these clinical problems could all negatively impact the quality of life of an HIV infected patient.
The effect of hypothyroidism on cardiovascular system has been greatly studied. Hypothyroidism increases vascular resistance, decreases left ventricular function and cardiac output. In, addition the decreased metabolic rate induced by hypothyroidism brings about cardiovascular risk factors such as dyslipidaemia and hypertension (7). These multiple cardiovascular pathways can reduce the quality of health and lead to decline in cardiovascular function.
Ukibe and colleagues [2017] demonstrated the presence of hypothyroidism and its impact on fertility in HIV infected females (9). Hypothyroidism indirectly leads to an increase in prolactin, which inhibits production and secretion of gonadotrophins. Thyroid hormones receptors are also expressed on oocytes, granulosa cells and theca cells, which also illustrates that thyroid hormones may have direct regulatory roles in menstrual cycle (10). Amongst the patients with hypothyroidism, serum levels of follicular stimulating hormone (FSH) and luteinizing hormone (LH) were significantly higher whilst progesterone and oestradiol levels were significantly lower; such hormonal abnormalities were explained to interfere with the sustenance of pregnancy and generate spontaneous abortion. Atis et al. [2010] have illustrated that the administration of FT4 to patients with hypothyroidism reverses menstrual abnormalities and increases spontaneous fertility (11). This may again point to the fact that it is vital to screen for hypothyroidism as it is one of the common thyroid condition in individuals living with HIV (5,12).
On the long-term, the ramifications of hypothyroidism have been studied in the context of subclinical hypothyroidism as opposed to overt hypothyroidism as it is usually treated. Overt hypothyroidism has also been reported by many researches, which further emphasise its prevalence amongst HIV patients (5,12).
Subclinical hypothyroidism
Subclinical hypothyroidism is a mild form of hypothyroidism that could progress to overt hypothyroidism. It presents as elevated TSH level associated with normal free thyroxine level (13). Thongam and colleague’s [2015] conducted cross-sectional study demonstrated the occurrence of subclinical hypothyroidism in 60 paediatric HIV type 1 (HIV-1) patients, and found 5 patients in both categories [30 taking highly active-antiretroviral therapy (HAART) and 30 HAART native]; had a thyroid function abnormality, irrespective of their use of HAART. Early discovery of such abnormalities is very imperative with children as it can negatively impact their growth (14). Same is also reported in adult HIV population and this can also be related to CD4 T-cell counts. This may suggest that thyroid abnormalities intensify with disease progression, thereby deterring quality of life at later stages of HIV. Although, subclinical hypothyroidism considered to be a mild clinical phenomenon and is usually asymptomatic; it is a sign of ongoing changes with thyroid gland that could either potentially progress to hypothyroidism or be an early sign of it (15-17).
Subclinical hypothyroidism induces depressed systolic function, left ventricular diastolic dysfunction, impairs vascular smooth muscle cell relaxation and causes arterial stiffness (16,17). These are important cardiovascular risk factors; patients with HIV have approximately 1.5–2-fold increased risk of developing acute myocardial infarction (AMI) (18,19). Therefore, interplay of subclinical hypothyroidism and HIV can be harmful.
Mechanism behind thyroid disease with HIV
Reports of hypothyroidism have been presented before the existence of HAART, which suggest that factors independent of the antiretroviral medication must be playing a part. Some literatures have stated that opportunistic infections namely, Pneumocystis jiroveci, tuberculosis, Coccidiodes, and Cryptococcus generate thyroiditis, thereby, hindering thyroid function (20-22). Alternatively, it has been found that drugs used to treat opportunistic infections could be the factor behind hypothyroidism in HIV; for example, Rifampin promotes reduction of peripheral thyroid hormone via hepatic microsomal enzymes (23,24). Although the occurrence of Kaposi sarcoma and lymphoma are rare or appear in the advanced stages of HIV, both conditions have been found to instigate malignant destruction of thyroid gland (25). Caloric deprivation and malnutrition have also been reported as a potential mechanism. Ricart-Engel et al. [1996] illustrated that malnourished HIV patients had low triiodothyronine (FT3) and reverse triiodothyronine (rT3) levels with normal FT4 and a constant FT3/rT3 ratio, they explained that hypoalbuminemia, a consequence of malnutrition, inhibits the transport of intracellular T4 into T3-producing organs (such as liver) (26). Therefore, FT3 levels become reduced due to reduced production. In addition, the common occurrence of selenium deficiency in malnourished HIV patients, leads to lack of selenium, which is necessary for deiodinase activity, hence, decreased FT3 and rT3 levels occur (27). Essentially, the knowledge we have on the underlying mechanism of thyroid disease in patients infected with HIV is not substantial. This could be due to the focus being diverted to antiretroviral medications, as increasing reports of hypothyroidism in patients receiving HAART are being made (Figure 1).
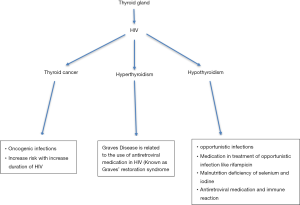
Role of antiretroviral in development of hypothyroidism
Since the introduction of HAART, thyroid dysfunction, particularly subclinical hypothyroidism, appears to be quite prevalent (28-30). Amongst untreated HIV infected patients’ prevalence of subclinical hypothyroidism is between 8–10%, whereas the prevalence is up to 12.6% amongst HAART users (31). Antiretroviral therapy usually involves the use of three medications in at least two different antiretroviral classes. Madeddu and colleagues [2002] illustrated that stavudine; a nucleoside reverse transcriptase inhibitor (NRTI) was being used by 80% of patients who had subclinical hypothyroidism or low FT4 levels (28). Other drugs in NRTI class such as zidovudine and lamivudine have not been associated with hypothyroidism (6). The World Health Organization (WHO) has recommended the disuse of stavudine due to other life-threatening side effects it induces. Research on its correlation with thyroid dysfunction stands relevant as stavudine is still being used in some countries due to the high cost of its alternatives and abundant stockpile (1) (WHO, 2013). Conversely, it has been suggested that there is no difference between the occurrence of hypothyroidism between HAART and HAART naïve patients. Particularly, the presentation of subclinical hypothyroidism in HAART patients has been deduced to being acute. Bongiovanni and colleagues [2006] illustrated that the occurrence of subclinical hypothyroidism in HIV infected patients utilizing HAART [stavudine, zidovudine and protease inhibitors (PI)] was most likely an acute phenomenon of using a new drug (32). In their study, the prevalence of subclinical hypothyroidism was higher amongst patients who had just started using HAART compared to those who had been on HAART for at least 1 year before the study. Drug interactions have been reported between Levothyroxine and antiretroviral drugs, especially protease inhibitors (PI), which could be due to shared glucuronidation (33,34). The mechanism behind the development of hypothyroidism and the progressive use of HAART remains unclear and necessitates further investigations. Therefore, it is important to maintain high index of clinical suspicion and screen for hypothyroidism according to the clinical assessment. It worth mentioning, that further longitudinal studies are needed about how HIV therapy may modulate the thyroid function.
Hyperthyroidism
Hyperthyroidism is a pathological disorder characterized by excessive thyroid secretion. Similar to hypothyroidism, hyperthyroidism can be overt or subclinical. Overt hyperthyroidism manifests due to reduced TSH concentrations and elevated FT4, FT3 or both. As the secretion of excessive thyroid hormone affects varied organs, symptoms such as weight loss, polydipsia, heat intolerance, tremor, palpitations, increased appetite and muscle weakness are often reported (3). Subclinical hyperthyroidism can be defined biochemically as low TSH with normal FT4 and FT3 levels (16).
Overt hyperthyroidism
Graves’ disease has been identified as the most common cause of hyperthyroidism in HIV infected individuals (35). It is an autoimmune disorder, which entails the activation of TSH receptors and consequent production and secretion of T4 and T3 due to the presence of stimulatory anti-TSH receptor antibodies (36). Development of Grave’s in HIV infected individuals is a complication of antiretroviral use and is sometimes called Graves’ immune restoration disease (GIRD) (37). Antiretroviral therapy helps suppress HIV replication, and enables restoration of naïve and memory CD4 T cells. The restoration of the immune system can become pathological and progress into immune reconstitution syndrome (IRIS), whereby the rapid repopulation of naïve T cells alongside failure to delete auto-reactive T cells in the thymus leads to organ-specific autoimmune disease. Thus, weeks to years after HAART has been introduced, production of autoantibodies including those to TSH receptors leads to development of Graves (36,37).
Hsu and colleagues [2016] reported cases of overt hyperthyroidism after patients were on antiretroviral therapy for years, ranging from 23 to 93 months. Amongst the 5 cases described, the patients had no risk factors or past history of thyroid disease (38). Prior to the introduction of HAART all the patients had very low CD4 T cells count and very high HIV viral load. In all the cases reported, immune regeneration had occurred by the time of hyperthyroidism diagnosis; their CD4 T cells had increased significantly and viral load had decreased substantially. For example, prior to the use of HAART by one of the patients’, CD4 count was 3 cells/µL (1%) whilst HIV viral load was 391,000 copies/mL with normal TSH level. Twenty-three months post antiretroviral therapy initiation; symptoms, signs and biochemical results characteristic of overt hyperthyroidism were positive. At this point immune restoration was evident as CD4 T cells count was 486 cells/µL (24%) and HIV viral load was undetectable. The clear presentation of Graves necessitates the importance of monitoring thyroid functioning as time goes and immune system begins to recover. Iordache et al. [2014] have reported that Graves’ can occur as early as 12 months after HAART initiation (39).
Hyperthyroidism can induce adverse complications that could make HIV infected patients very unwell such as ophthalmopathy, dermopathy and cardiovascular problems. Ophthalmopathy occurs in 25% of Graves’ disease patients; signs such as proptosis, diplopia and periorbital oedema are seen (40). Edmunds et al., 2015 report cases of ophthalmopathy amongst HIV infected patients being treated for Graves’ disease and further highlight challenges in managing ophthalmopathy-Graves’-HIV co-existence (36). Overt hyperthyroidism is most commonly associated with non-NNRTI, especially efavirenz (37). HAART medications may also interact with the metabolism of medications required to manage ophthalmopathy, NNRTIs are cytochrome P450 (CYP450) inducers, and whilst PI-boosted by ritonavir are CYP450 inhibitors (36). In terms of preparation for surgical interventions ritonavir may increase toxic metabolites of pethidine but decrease plasma concentrations of morphine. Ritonovir may also increase plasma levels of hypnotics such as fentanyl or midazolam and cause Cushing’s syndrome due to the potentiating effects it has on steroids (41). Specific PIs such as saquinavir interacts with fentanyl and alfentanil to cause ventricular arrhythmia (36). Therefore, the need to consider all the points mentioned above before starting management of hyperthyroidism in individuals LWHIV. The surgical management of hyperthyroidism ideally should be discussed between HIV specialist, endocrinologists and surgeons, in order to decrease complications (Figure 1).
Subclinical hyperthyroidism
The progression of subclinical hyperthyroidism is quite diverse in HIV patients, thus, there are controversies on the benefit of its treatment. Its occurrence could, precede overt hyperthyroidism; remain stable for a long period or even reverse to normal thyroid functioning. Subclinical hyperthyroidism has been shown to decrease quality of life scores in HIV negative young and middle-aged patients (42). The life-threatening risks on cardiovascular system such as atrial fibrillation and stroke, suggests the need for increased monitoring amongst HIV infected patients, as they are already vulnerable.
Thyroid malignancy
Within HIV infected population the incidence of non-acquired immune deficiency syndrome (non-AIDS) defining malignancies is steadily increasing compared to that of AIDS. There are cases of clinically silent non-AIDS malignancies being revealed at autopsy (43). Four incidental cases of papillary thyroid carcinoma (PTC) were found in a Brazilian autopsy study exploring thyroid abnormalities in HIV infection (43). A few clinical cases of thyroid cancer diagnosis amongst HIV infected patients have been reported also. Manfredi et al., [2015] reported a unique case of a patient with HIV who developed 4 non-AIDS malignancies within the stretch of 12 years, of which one was PTC (papillary thyroid carcinoma) (44). Furthermore, Linares et al. [2010] published a case report on the manifestation of micrometastatic PTC in an individual that had a well-controlled HIV infection (45). Medullary thyroid cancer has also been diagnosed in an HIV infected individual (46). Additionally, follicular thyroid cancer has been found in a long-term non-progressor HIV infected patient (47) (Phatak et al., 2015). In these clinical cases, none of the patient had the classical risk factors of thyroid cancer.
As the prevalence of thyroid cancer is yet to be analysed, it is difficult to draw conclusions if there is a link between HIV and thyroid cancers or if HIV is the main risk factor. However, there has been some research on incidence of other solid tumours in HIV infected patients. Whereby, the occurrence of non-AIDS malignancies has been attributed to the continuous imbalance between concurrent oncogenic infections, HIV infection itself and lifestyle habits with the recovering CD4 T cells count. It has also been recognised that the risk of cancer development increases with duration of HIV infection (Figure 1).
Conclusions
Thyroid abnormalities in patients with HIV is unique due to the fact that treatment of HIV is associated with dyslipidemia which in turn may lead to an increase in cardiovascular risks. Furthermore; after introduction of HAART, production of auto-antibodies including those to TSH receptors leads to development of Graves’ disease. Importantly, in well treated individuals LWHIV, no changes in thyroid functions were reported (48). Due to the fact that the epidemiology of thyroid malignancy in HIV infected individuals and the cases reported are very few, further research is required to understand the association between HIV and thyroid malignancy. Importantly, further research will also allow to gain an understanding if thyroid screening will be required in routine basis for individuals LWHIV.
Acknowledgments
Dr. Ahmed would like to acknowledge the support of the staff in ward 3 and Blood Borne Virus department in Milton Keynes University Hospital, UK.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2019.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization (WHO). Global Health Observatory data: Number of deaths due to HIV. 2017. Available online: http://www.who.int/gho/hiv/epidemic_status/deaths/en/. Accessed 13th August 2018.
- Grinspoon SK, Donovan DS, Bilezikian JP. Aetiology and pathogenesis of hormonal and metabolic disorders in HIV infection. Baillieres Clin Endocrinol Metab 1994;8:735-55. [Crossref] [PubMed]
- Mylonakis E, Koutkia P, Grinspoon S. Diagnosis and treatment of androgen deficiency in human immunodeficiency virus—infected men and women. Clin Infect Dis 2001;33:857-64. [Crossref] [PubMed]
- Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 1998;12:F51-8. [Crossref] [PubMed]
- Ji S, Jin C, Höxtermann S, et al. Prevalence and influencing factors of thyroid dysfunction in HIV-infected patients. Biomed Res Int 2016;2016:3874257. [Crossref] [PubMed]
- Collazos J, Ibarra S, Mayo J. Thyroid hormones in HIV infected patients in the highly active antiretroviral therapy era: evidence of an interrelationship between the thyroid axis and the immune system. AIDS 2003;17:763-5. [Crossref] [PubMed]
- Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet 2017;390:1550-62. [Crossref] [PubMed]
- Beltran S, Lescure FX, Desailloud R, et al. Increased prevalence of hypothyroidism among human immunodeficiency virus-infected patients: a need for screening. Clin Infect Dis 2003;37:579-83. [Crossref] [PubMed]
- Ukibe NR, Ukibe SN, Emelumadu OF, et al. Impact of thyroid function abnormalities on reproductive hormones during menstrual cycle in premenopausal HIV infected females at NAUTH, Nnewi, Nigeria. PLoS One 2017;12:e0176361. [Crossref] [PubMed]
- Zhang SS, Carrillo AJ, Darling DS. Expression of multiple thyroid hormone receptor mRNAs in human oocytes, cumulus cells, and granulosa cells. Mol Hum Reprod 1997;3:555-62. [Crossref] [PubMed]
- Atis G, Dalkilinc A, Altuntas Y, et al. Sexual dysfunction in women with clinical hypothyroidism and subclinical hypothyroidism. J Sex Med 2010;7:2583-90. [Crossref] [PubMed]
- Noureldeen AF, Qusti SY, Khoja GM. Thyroid function in newly diagnosed HIV-infected patients. Toxicol Ind Health 2014;30:919-25. [Crossref] [PubMed]
- Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs 2012;72:17-33. [Crossref] [PubMed]
- Thongam S, Keithelakpam S, Singh TY, et al. Thyroid dysfunction in human immunodeficiency virus-infected children and its correlation with CD4(+) T lymphocyte count. Indian J Endocrinol Metab 2015;19:272-6. [Crossref] [PubMed]
- Emokpae MA, Akinnuoye IM. Asymptomatic thyroid dysfunction in human immunodeficiency virus-1-infected subjects. J Lab Physicians 2018;10:130-4. [PubMed]
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012;379:1142-54. [Crossref] [PubMed]
- McDermott MT. Euthyroid sick syndrome. In: Endocrine Secrets. Sixth edition. Philadelphia: Saunders, 2014:314-7.
- Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506-12. [Crossref] [PubMed]
- Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep 2013;10:199-206. [Crossref] [PubMed]
- Zavascki AP, Maia AL, Goldani LZ. Pneumocystis jiroveci thyroiditis: report of 15 cases in the literature. Mycoses 2007;50:443-6. [Crossref] [PubMed]
- Kiertiburanakul S, Sungkanuparph S, Malathum K, et al. Concomitant tuberculous and cryptococcal thyroid abscess in a human immunodeficiency virus-infected patient. Scand J Infect Dis 2003;35:68-70. [Crossref] [PubMed]
- Jinno S, Chang S, Jacobs MR. Coccidioides thyroiditis in an HIV-infected patient. J Clin Microbiol 2012;50:2535-7. [Crossref] [PubMed]
- Sellmeyer DE, Grunfeld C. Endocrine and metabolic disturbances in human immunodeficiency virus infection and the acquired immune deficiency syndrome. Endocr Rev 1996;17:518-32. [PubMed]
- Etzel JV, Brocavich JM, Torre M. Endocrine complications associated with human immunodeficiency virus infection. Clin Pharm 1992;11:705-13. [PubMed]
- Parsa AA, Bhangoo A. HIV and thyroid dysfunction. Rev Endocr Metab Disord 2013;14:127-31. [Crossref] [PubMed]
- Ricart-Engel W, Fernández-Real JM, González-Huix F, et al. The relation between thyroid function and nutritional status in HIV-infected patients. Clin Endocrinol (Oxf) 1996;44:53-8. [Crossref] [PubMed]
- Contempré B, Duale NL, Dumont JE, et al. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin Endocrinol (Oxf) 1992;36:579-83. [Crossref] [PubMed]
- Madeddu G, Spanu A, Chessa F, et al. Thyroid function in human immunodeficiency virus patients treated with highly active antiretroviral therapy (HAART): a longitudinal study. Clin Endocrinol (Oxf) 2006;64:375-83. [PubMed]
- Calza L, Manfredi R, Chiodo F. Subclinical hypothyroidism in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002;31:361-3. [Crossref] [PubMed]
- Grappin M, Piroth L, Verges B, et al. Increased prevalence of subclinical hypothyroidism in HIV patients treated with highly active antiretroviral therapy. AIDS 2000;14:1070-2. [Crossref] [PubMed]
- Silva GA, Andrade MC, Sugui Dde A, et al. Association between antiretrovirals and thyroid diseases: a cross-sectional study. Arch Endocrinol Metab 2015;59:116-22. [Crossref] [PubMed]
- Bongiovanni M, Adorni F, Casana M, et al. Subclinical hypothyroidism in HIV-infected subjects. J Antimicrob Chemother 2006;58:1086-9. [Crossref] [PubMed]
- Touzot M, Beller CL, Touzot F, et al. Dramatic interaction between levothyroxine and lopinavir/ritonavir in a HIV-infected patient. AIDS 2006;20:1210-2. [Crossref] [PubMed]
- Lanzafame M, Trevenzoli M, Faggian F, et al. Interaction between levothyroxine and indinavir in a patient with HIV infection. Infection 2002;30:54-5. [Crossref] [PubMed]
- Sheikh V, Dersimonian R, Richterman AG, et al. Graves' disease as immune reconstitution disease in HIV-positive patients is associated with naive and primary thymic emigrant CD4(+) T-cell recovery. AIDS 2014;28:31-9. [Crossref] [PubMed]
- Edmunds MR, Mellington F, Ford RL, et al. Clinical challenges of thyroid eye disease in HIV-positive patients on highly active antiretroviral therapy. J Clin Endocrinol Metab 2015;100:779-87. [Crossref] [PubMed]
- Rasul S, Delapenha R, Farhat F, et al. Graves' Disease as a Manifestation of Immune Reconstitution in HIV-Infected Individuals after Initiation of Highly Active Antiretroviral Therapy. AIDS Res Treat 2011;2011:743597. [Crossref] [PubMed]
- Hsu E, Phadke VK, Nguyen ML. Short Communication: Hyperthyroidism in Human Immunodeficiency Virus Patients on Combined Antiretroviral Therapy: Case Series and Literature Review. AIDS Res Hum Retroviruses 2016;32:564-6. [Crossref] [PubMed]
- Iordache L, Launay O, Bouchaud O, et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev 2014;13:850-7. [Crossref] [PubMed]
- De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet 2016;388:906-18. [Crossref] [PubMed]
- Foisy MM, Yakiwchuk EM, Chiu I, et al. Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med 2008;9:389-96. [Crossref] [PubMed]
- Biondi B, Palmieri EA, Fazio S, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab 2000;85:4701-5. [PubMed]
- Basílio-De-Oliveira CA. Infectious and neoplastic disorders of the thyroid in AIDS patients: an autopsy study. Braz J Infect Dis 2000;4:67-75. [PubMed]
- Manfredi R, Cascavilla A, Magistrelli E, et al. A patient with a 12-year history characterized by four non-AIDS-related malignancies, occurring before and after the disclosure of HIV infection. Eur J Epidemiol 2015;30:459-61. [Crossref] [PubMed]
- Lloret Linares C, Troisvallets D, Sellier P, et al. Micrometastasis of papillary thyroid carcinoma in a human immunodeficiency virus-infected patient: a case report and discussion. Med Oncol 2010;27:756-9. [Crossref] [PubMed]
- Isik M, Gokcay A, Altundag K. Thyroid medullary carcinoma in a patient with HIV/AIDS. Int J Hematol Oncol 2012;27:192-4. [Crossref]
- Phatak UA, Chitale PV, Jagdale RV. Thyroid cancer in a long-term nonprogressor HIV-1 infection. Indian J Sex Transm Dis AIDS 2015;36:195-7. [Crossref] [PubMed]
- Harsløf M, Knudsen AD, Benfield T, et al. No evidence of increased risk of thyroid dysfunction in well treated people living with HIV. AIDS 2018;32:2195-9. [Crossref] [PubMed]
Cite this article as: Ibrahim M, Adeniran A, Ibrahim MA, Woodward C, Mital D, Ahmed MH. Thyroid function in individuals living with human immunodeficiency virus: the concern and debate about regular screening. Ann Thyroid 2019;4:12.


