The evolution and progress of standard procedures for intraoperative nerve monitoring
Introduction
The recurrent laryngeal nerve (RLN) and external branch of superior laryngeal nerves (EBSLN) are at risk of injury during all thyroid and parathyroid surgeries by virtue of their close proximity to the operative field and injury to these nerves can result in significant patient morbidity secondary to the voice, swallowing and breathing issues that nerve dysfunction triggers. During neck endocrine surgeries, rates of nerve injury have traditionally been reported as low (3–5%). However, more recent series suggest that these past reports have significantly underestimated the true incidence, citing rates closer to 10%. Similarly, although actual incidence of EBSLN injury remain unknown, it may approach 58% (1,2). These inconsistencies in reported injury rates likely relate to many factors including a lack of standardization of pre and post-operative laryngeal examination practices, the often subtle and variable nature of nerve palsy symptoms, and reporting biases from large thyroid centers where complication rates are low. Clinical implications of unilateral RLN injury revolve around breathing, phonation, and swallowing with severity ranging from mild impairment to severe. Conversely, bilateral RLN injury can necessitate the placement of a tracheostomy by causing a grossly narrowed, immobile larynx and significant respiratory distress. Clinical symptoms of EBSLN injury are variable, often may be overlooked, but can be significant, especially to professional voice-users. The symptoms include vocal fatigue and decreased pitch and inability to project voice. The laryngoscopy findings associated with EBSLN injury include posterior glottic rotation toward the paretic side, bowing of the vocal fold on the weak side and inferior displacement of the affected cord. Notably, these findings can be subtle and can easily go unnoticed.
Techniques to identify and monitor the laryngeal nerves during neck endocrine procedures have been developed over the past 50 years with the aim of minimizing postoperative nerve dysfunction and thus lowering patient morbidity. Many techniques have been proposed, from simple nerve identification to intermittent and, now, continuous nerve monitoring. Specialized committees have been formed and guidelines have been published to encourage standardization of monitoring techniques. This has helped in uniform collection of monitoring data across different populations of patients and surgeons, which along with technical advances in neuromonitoring techniques and better understanding of neurophysiology of the larynx have been instrumental in continued evolution of monitoring strategies and management algorithms. This article discusses the evolution of these monitoring techniques over the past five decades and highlights the current international recommendations for neural monitoring in neck endocrine surgery. Future directions in nerve monitoring will also be discussed.
Evolution of intraoperative nerve monitoring (IONM)
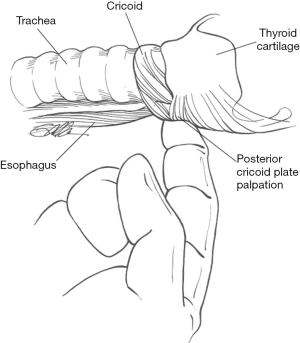
One of the earliest reports of electrical RLN and EBSLN identification, stimulation and response evaluation was in 1966 in the Annals of Surgery. This study used a canine model intubated with an endotracheal tube (ET) with a balloon placed within the larynx and connected to a pressure-recording system. Results indicated that the pressure changes from an intralaryngeal balloon consistently showed changes upon RLN electrical stimulation and, for the first time, provided a method for electrical identification of the RLN. Similar changes were noted for the SLN. The same group translated this research to the human population during thyroid surgeries, showing that endolaryngeal balloon pressure recording could indicate when the RLN and SLN were stimulated (3). In 1970, Riddell published a 23-year series spanning from 1946 to 1969, reporting on RLN identification using laryngeal palpation with simulation of the RLN as an additional safety measure for nerve protection during thyroid surgery (4). In 1986, palpation of the posterior cricoarytenoid muscle with nerve stimulation of 0.5–2.0 mA was presented as a simple and safe technique for RLN identification and assessment during thyroid surgeries, in addition to a thorough knowledge of cervical anatomy (5).
Over the past thirty years, different IONM techniques have been proposed including laryngeal palpation (Figure 1), glottic pressure monitoring, glottic observation, intralaryngeal hookwire electrodes, ET based electrodes and post cricoid surface electrodes. Of these, ET based surface electrodes (Figure 2) are currently the most popular for monitoring of the RLN in thyroid surgery and have several advantages over other methods including ease of set up, non-invasive nature and large EMG potentials recordable with such electrodes. However as with any neurophysiological monitoring category, whether intracranial, spinal or peripheral, standardization of IONM technique is necessary to ensure that results generated are repeatable, reliable and clinically meaningful. Studies investigating the utility of IONM compared to direct nerve visualization alone have varied greatly with regards to inclusion criteria, laryngeal examination practices, type of monitoring applied and reported results. Most studies have used ET based surface electrodes to record electromyographic potentials elicited by vocal fold contraction in response to intermittent direct nerve stimulation. More recently, there have been a number of series reporting methods of continuous intraoperative nerve monitoring (CIONM) which has the advantage over intermittent stimulation techniques of assessing real-time nerve integrity and thus better preventing impending nerve injury. Data is lacking as to whether CIONM will ultimately reduce temporary and/or permanent RLN injury rates however the concept, by virtue of its potential preventative benefits, is appealing.
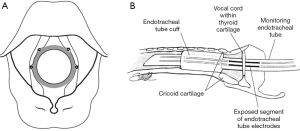
It is important to remember that a key element in the anesthetic technique with IONM is the avoidance of muscle relaxants or the use of muscle relaxants with a very short half-life for endotracheal intubation. In general, to date the research and documentation involving the anesthetic techniques for IONM are limited. As IONM techniques and applications continue to evolve, the need for a collaborative environment between the surgeon and anesthesia services leading to optimal IONM for better surgical outcomes is increasing. Macias et al have published an interdisciplinary collaborative protocol for monitored neck surgery based on the evidence and clinical experience of over 3,000 cases (6).
Uptake of IONM worldwide
There has been increasing interest in IONM over the past decade as outcome measures assume greater importance in patient care algorithms and as more practitioners recognize that macroscopic nerve identification and preservation does not necessarily equate to functional neural integrity (7-13). In 2006, the American Association of Endocrine Surgeons (AAES) evaluated attitudes about neuromonitoring, usage patterns and predictors of its use via email surveys. Non users of IONM tended to be older (P=0.023) with a lower case volume (P=0.003), less familiarity with the technology (P<0.001), and less access to the equipment (P<0.001) (8). However, despite these findings, the percentage of surgeons in the United States who always use nerve monitoring continues to increase particularly amongst young surgeons, academic surgeons and those in high volume centers. In Germany, IONM is standard of care and, according to a national survey in 2010, 90% of surgical departments in Germany are equipped with nerve monitors with RLN monitoring used in 93% of thyroidectomies (10,14). In the United Kingdom, a minority of members of the British Association of Endocrine and Thyroid Surgeons (BAETS) use IONM, however this proportion is gradually increasing (11). In a 2005 study from the UK, patient age did not impact on the use of IONM however type of disease present (malignant versus benign) and timing of surgery (revision versus primary procedures) were both significantly correlated with IONM usage (P<0.00001) (12). In Denmark, an IONM usage rate of 77% has been reported from a registry of surgical results in 2007 (13) and similarly, in France, IONM use increased from 6,200 in 2008 to 10,000 in 2010. Reports out of Italy, Poland, Spain and China have reported similar utilization trends. A survey from Italy identified that the main motivations for IONM use included medico-legal reasons (30%), RLN confirmation (20%), RLN identification (20%), prognosis (10%) difficult cases (10%), decreased surgical time (5%) and education (5%). High volume and academic centers reported use more consistent with published guidelines of IONM (15). The recent world-wide standards and recommendations published in guidelines by various organizations are described in the “Current Standards for IONM in thyroid and parathyroid surgeries section” below.
IONM in the reduction of RLN injury
The end-points of most published series looking at IONM in neck endocrine surgeries are transient and permanent RLN injury. However, the ability of many trials to demonstrate a clear benefit of IONM over no monitoring is limited by heterogeneity as well as lack of adequate power in study design. This heterogeneity is due to many factors including variability in pre- and post-operative laryngeal examination practices, utilization of different formats of IONM (e.g., invasive versus noninvasive devices, audio versus audio plus EMG documentation), sample size limitations, histologic nature of the pathology, type and extent of the surgical procedure, and surgeon’s expertise. Dralle et al. have shown that it would be very difficult to have an adequately powered study to differentiate between visual identification alone and IONM, as such a study would require 9 million nerves at risk (NAR) in benign goiter patients and 40,000 NAR in thyroid cancer patients per arm to detect statistical significance (16). One of the larger studies performed to assess the value of IONM in neck endocrine procedures was by Barczyński et al. (17). In over 850 patients undergoing revision thyroidectomy, there was a statistically significant reduction in transient (2.6% versus 6.3%) and permanent (1.4% versus 2.4%) paralysis rates between patient receiving IONM and those without IONM. Another study by Barczyński et al. compared the outcomes of 1,000 patients with IONM versus 1,000 patients without IONM (18). They found that there was a lesser prevalence of transient nerve damage in the IONM group among high-risk patients. Additionally, there was a difference between low-risk and high-risk patients which did not reach statistical significance. Overall conclusions from this prospective study were that IONM, compared to visualization alone, reduces the incidence of transient, but not permanent, nerve injury. Thomusch et al. studied over 5,000 procedures and concluded that IONM of the RLN in thyroid surgery significantly (P<0.05) lowers rates of transient and permanent RLN palsy compared to visual RLN identification alone (19). Yet other studies have demonstrated that IONM can reduce operative time and related costs but virtue of less time taken to identify the RLN.
A meta-analysis by Zheng et al. analyzed 5 prospective randomized studies and 12 comparative non-randomized studies (20). The global sample was >36,000 NAR. Transient neural damage was significantly reduced in the IONM group (2.56%) compared with 2.71% without IONM however permanent events showed no statistical significance. Pisanu et al. analyzed 3 prospective studies and 17 comparative non-randomized trials with a global sample of >35,500 NAR and reported similar RLN paralysis rates between with-IONM and without-IONM groups (21). Sanabria et al. analyzed prospective trials only with a global sample of >3,000 NAR and noted no statistically reduced rates of temporary or permanent RLN paralysis with IONM, although there were trends towards such significance in both groups (22).
Important applications of IONM
The intraoperative assessment of RLN function with IONM during thyroid surgery is uniquely advantageous for many reasons, some of which are listed below as a lead-in to the subsequent discussion on current guidelines and standards for IONM in thyroid and parathyroid surgeries.
- Early nerve identification and mapping.Particularly during complicated thyroid operations, early RLN identification can help avoid inadvertent nerve injury. Multiple studies have shown that IONM improves visual nerve identification rates by allowing for the nerve path to be mapped using probe stimulation at 2 mA well before visual identification is possible. Once the nerve is visually identified, stimulation at a current of 1 mA allows for mapping of the nerve.
- To identify anatomical variations such as extralaryngeal branches, specifically the motor branches, as well as non-RLN (23). It also identifies distortions in the nerve position caused by the primary thyroid disease process (e.g., substernal extension of a large thyroid goiter).
- Assistance in optimizing tissue removal during total thyroidectomy by facilitating dissection of tissues in the region of the ligament of Berry.
- Intraoperative detection of impending neuropraxic neural injury likely allowing alteration of causative surgical maneuvers. It is important to realize that IONM is more useful in stretch or compression injury than in transactional nerve injury.
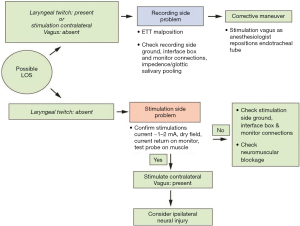
- Prognostication of postoperative neural function, this assumes particular importance in bilateral surgeries, for prevention of bilateral vocal fold paralysis (BVFP). IONM can prevent bilateral RLN palsy associated with total thyroidectomy by alerting the surgeon to consider a staged procedure when ipsilateral LOS occurs. Standardized LOS troubleshooting algorithms have been developed to assist that the surgeon using IONM in identifying the true LOS and determining optimal course for any remaining parts of the surgical procedure.
- Improved rates of identification of superior laryngeal nerve.
- Educational and medicolegal reasons.
- For low volume surgeons, RLN monitoring may significantly decrease the frequency of permanent RLN paralysis (24).
Current standards for IONM in thyroid and parathyroid surgeries
There is increasing organizational support for IONM in neck endocrine surgeries, catalyzed at least in part by the introduction of non-invasive monitoring devices, publication of guidelines defining standards for RLN/EBSLN monitoring and of randomized prospective trials as well as by establishment of structured training courses. The American Academy of Otolaryngology and Head and Neck Surgery (AAOHNS) guidelines on voice preservation during thyroid surgery state that IONM is an option for patients undergoing thyroid surgery in order to (I) reduce RLN identification time, (II) decrease temporary VCP rates, and (III) avoid bilateral VCP (through prognostication of postoperative vocal cord function). These guidelines suggest significant utility of IONM in cases of (I) bilateral thyroid surgery, (II) revision thyroid surgery, and (III) surgery in the setting of an existing RLN paralysis (25). Similarly, guideline statements from the American Head and Neck Society (AHNS) on recurrent thyroid carcinoma (26), central neck dissection (27), and invasive thyroid carcinoma (28) concur that IONM is a valuable tool to assist with nerve identification, maximizing nerve functional integrity and prognostication of postoperative laryngeal function. The American Thyroid Association (ATA) has made mention of IONM within a number of consensus statements and guidelines. Recommendation 42 of the ATA 2015 Guidelines for Thyroid Nodules and Well Differentiated Thyroid Cancer (29) state that visual identification of the RLN during dissection is required in all cases and that intraoperative neural stimulation (with or without monitoring) may be considered to facilitate nerve identification and to confirm neural function”. In addition, they note that training and observation of existing monitoring standards are important to provide optimal benefit from IONM given the complexity of monitoring systems. Two additional ATA Surgical Affairs Committee consensus statements (on outpatient thyroid surgery and on optimal surgical management of goiters) note that neural monitoring can be helpful in confirming intact neural function at the end of surgery, which may impact on discharge planning (30,31). Both the German Association of Endocrine Surgery and the International Neuromonitoring study group (INMSG) recommend neural monitoring in all cases of thyroid and parathyroid surgery.
The International Neural Monitoring Study Group (INMSG) was founded in 2006 to serve the emerging field of neurophysiologic monitoring of laryngeal nerves in neck endocrine surgery. It is an international multidisciplinary collaboration with experts in the field of neck endocrine surgery, laryngology, electromyography, anesthesiology, and neurophysiology. The goals of this collaborative group are to improve the quality of IONM, reduce inappropriate variations in IONM technique, adhere to strict standardization, foster the growth and stature of neurophysiological monitoring, encourage research, improve and update guidelines, implement IONM courses, develop quality standards for practice and training, define unequivocal references of RLN neurophysiology and pathology, refine EBSLN monitoring and evaluate new developing technology such as CIONM. They have published guidelines on RLN and EBSLN IONM standards for monitored thyroid and parathyroid surgery (32,33). The standards described in these guidelines brought uniformity in application of IONM during endocrine neck surgery. Since the publication of these guidelines, several papers documenting normative electromyography (EMG) data of the vagus nerve, RLN and EBSLN have been published (34-36).
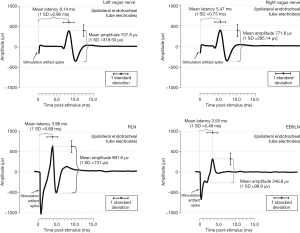
To recognize abnormal EMG responses and to meaningfully interpret the EMG responses recorded by IONM, establishing normal range of EMG responses for Vagus nerve, RLN and EBSLN is important. Sritharan et al. (35) have delineated normative waveform morphology, mean latency and mean amplitude of the left and right vagus nerve, pooled RLN and pooled EBSLN (Figure 3). Caragacianu et al. have proposed an intraoperative EMG criteria, which when present imply normal vocal cord function postoperatively (37).
More recently INMSG has written the soon to be published, a two-part consensus guideline discussing nerve monitoring for neck endocrine procedures. These documents contain an extensive discussion of staging bilateral thyroid surgery with monitoring loss of signal (LOS) (Part I) and of optimal RLN monitoring for invasive thyroid cancer (Part II). These are explicitly detailed documents and represent the most expansive guideline statements to date with evidence based literature reviews and technical discussions. They greatly help with the standardization of current techniques and clarify potential pitfalls of IONM in thyroid and parathyroid surgeries.
Part I of the INMSG guidelines discusses the use of IONM in bilateral thyroid surgery. BVFP results in tracheostomy in approximately 30% of patients. IONM provides a way of determining whether a nerve exhibits a LOS, thus potentially preventing bilateral BVFP by allowing the surgical procedure to be staged. The definition of LOS was proposed by the INMSG in 2011 as an EMG signal with amplitude of 100 µV or less. For this criteria to be used, initial EMG amplitude should be 500 µV or greater. Most LOS injuries occur due to traction, especially around the ligament of Berry. However, few of these injuries will be visually evident intraoperatively. Although recently there has been a movement towards unilateral thyroid surgery in low risk cancers up to 4 cm, rates of total thyroidectomy continue to increase in the US and around the world, and all surgeons—regardless of caseload volume—have increased complications with total as compared to hemi thyroidectomy. Interestingly, contralateral cord paralysis after ipsilateral LOS appears to higher than rates where no ipsilateral LOS occurs, likely due to patient and surgeon related factors. Thus, when LOS is incorporated into the surgical strategy by staging a planned bilateral thyroidectomy, rates of bilateral VCP and tracheotomy can be dramatically reduced (Figure 4). The cost effectiveness of neural monitoring and LOS/staged thyroidectomy has also been shown to be favorable. The Part I document also introduces a ‘yellow-red-black’ type model to represent the various stages of possible RLN injury. EMG-Yellow reflects impending neuropraxia with amplitude decrease of 50% or more and latency increase of 10% or more, the so-called ‘combined event’. These changes are often reversible with alteration of the surgical maneuver. EMG-Red reflects evolving neuropraxia with a further increase in the risk of VCP from the ‘yellow’ stage that occurs as the EMG amplitude drops to 100 µV or less. The Final EMG stage is the F-EMG-Black whereby the final EMG is considered as recovery EMG if both an absolute value of 250 µV along with recovery to >50% of baseline amplitude are present. Laryngeal twitch response to nerve stimulation may also be useful to support final LOS.
Part II of the INMSG guidelines discusses optimal RLN monitoring for invasive thyroid cancer. The RLN is one of the most frequent sites of invasion for locally aggressive thyroid cancer. RLN dysfunction from carcinoma invasion often occurs insidiously and, preoperatively, may present with voice and/or aspiration related issues or may be asymptomatic. Thus, to identify possible RLN paralysis pre-operatively, laryngeal examination should be performed on all patients with (I) with preoperative voice abnormality (II) a past history of surgery that may have endangered the vagus or RLN or (III) all patients with potentially invasive primary thyroid disease or significant central neck nodal disease. Cross sectional imaging can further define the potential degree of nerve involvement when VCP is detected on laryngoscopy preoperatively. Different types of RLN invasion are possible including perineural invasion involving only the epineurium (shaving of tumor may be possible in these cases), and perineural invasion with additional invasion into perineurium and endoneurium (nerve resection may be indicated in these cases depending on additional factors as detailed in these guidelines). EMG information obtained intraoperatively can help guide decision making in the invaded nerve and assist in determining when nerve preservation versus sacrifice is indicated. For nerves with preservation of some glottic function or proximal RLN EMG activity, consideration should be made to preserving the nerve whenever possible.
CIONM
Although intermittent IONM is extremely helpful, it can only provide intermittent RLN evaluation, allowing the nerve to be at risk of injury in between the stimulus. Hence, a new format of IONM that uses an implanted vagal electrode (Figure 5) to constantly provide a real time intraoperative EMG data of vagus nerve and RLN has evolved, it is termed continuous IONM (CIONM) (38-40). The adverse EMG changes detected by CIONM can indicate an impending neural injury and hence allow the surgeons to perform a corrective action like stopping/reversing underlying maneuvers that may have caused the adverse EMG changes, thus may be able to avoid a permanent injury. Phelan et al. analyzed EMG adverse events and have coupled amplitude decline and latency incline to identify mild combined events (mCE) and severe combined events (sCE). They defined mild combined event (mCE) as concordant amplitude decrease of 50–70% and a latency increase of 5–10% and sCE as concordant amplitude decrease of >70% and a latency increase of >10%. The study noted that mCE was not related to postoperative VCP, whereas the sCE could evolve into frank LOS, which were much less reversible and could lead to postoperative VCP.
Current standards for EBSLN monitoring
Evolution of EBSLN monitoring deserves special mention as it has been a much more recent addition to the IONM strategies. True rates of EBSLN injury after neck endocrine procedures are unknown but may approach 50% in some series. In part, this is because objective examination findings of such injury are often difficult to discern, even with direct laryngeal visualization. Voice changes after injury to the EBSLN can range in severity from mild to severe and causes altered fundamental frequency of the voice, difficulty producing high frequency sounds, and reduced vocal projection. However, due to the paucity of examination findings and lack of changes in vocal fold mobility, these changes are often overlooked and patients are reassured and discharged from follow up without further voice evaluation. EMG of the cricothyroid muscle (CTM) is the gold standard examination for diagnosis of EBSLN injury. Multiple classification schemes have been proposed to aid in EBSLN identification during thyroidectomy procedures, including the Cernea, Kierner, Friedman and Selvan EBSLN Classification Schemes (41-43).
Until recently, there were few reports in the literature describing how to identify and preserve the EBSLN during superior pole dissection. Most surgeons tend to avoid rather than routinely expose the EBSLN during thyroidectomy and reports on methods of EBSLN preservation in the literature have thus varied from visualization alone to electrical stimulation (44-48), although most surgeons tend to avoid rather than routinely expose and identify the EBSLN during thyroidectomy. In 2013, to improve the practice of neural monitoring of the EBSLN during thyroidectomy and parathyroidectomy, the INMSG published a guideline statement on EBSLN monitoring during neck endocrine surgery which introduced standardized procedures for EBSLN identification and stimulation (33).
As compared to IONM of the RLN, EBSLN monitoring is based on two distinct outcome measures, namely (I) evaluation of the cricothyroid twitch response (present in all patients); and (II) EMG glottic response of vocal cord depolarization identified on surface ET electrodes. This is present in 70–100% of patients depending on type of ET used, notably, the Trivantage tube (Figure 6) has higher sensitivity (49). Meticulous dissection of the superior thyroid pole is essential to ensure the EBSLN is not entrapped within branches of the superior vascular pedicle prior to vessel ligation and division. Approximately 20% of EBSLNs may not be able to be visually identified due to a subfascial/intramuscular course within the inferior constrictor muscle (44) however the INMSG guidelines recommend that attempts to directly visualize the EBSLN should be made in all cases. The cricothyroid twitch technique for EBSLN IONM is based on the following premises:

- The EBSLN needs to be stimulated as clearly presents (through CTM visual twitch assessment or endotracheal glottic waveform if observable) cranially and medially to the evolving superior pole pedicle (true positive stimulation);
- Stimulation of the pedicle that is to be divided is stimulated as negative for EBSLN (i.e., no CTM visual twitch or endotracheal glottic waveform) (true negative for absence of neural tissue in the pedicle provided that a true positive stimulation is initially obtained).
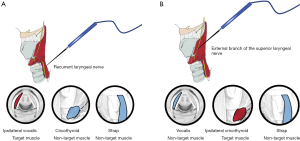
The Stimulation-Glottic EMG technique tor EBSLN IONM uses ET electrodes to measures the glottic depolarization resulting from ipsilateral EBSLN stimulation. This is thought to occur due to the human communicating nerve which is an extension of the EBSLN that innervates the anterior half of the ipsilateral vocal fold, described in up to 85% of anatomical dissection studies (50). Equipment-related measurement issues may explain why an identifiable glottic waveform is present in only 70–80% of patients using standard EMG tubes. False positive stimulation of the EBSLN occurs when a positive CTM waveform is obtained due to a nonneural shunt stimulation and may occur when 2 mA stimulation currents are utilized during nerve mapping. False positive results can be minimized by reducing stimulation current to 1mA following nerve identification and observing for a positive CTM twitch response. Liddy et al. (51) have studied correlation of diverse EMG response with presence or absence of visual and palpable muscle activation to document target muscles (those with concomitant muscular contraction) and non-target muscles (those with no concomitant muscular contraction) for RLN and EBSLN (Figure 7A,B). They noted that recording low-amplitude EMG waveform in non-target muscles when a nerve is stimulated can be explained by understanding the far-field artifactual waveforms concept. False negative results occur when the EBSN is misidentified for a nonneural structure, resulting in no CTM twitch (and no EMG response) following EBSLN stimulation, and may result from equipment related recording issues, blood or fascial covering over the nerve, insufficient stimulation current, neuromuscular blockade, and transient EBSLN neuropraxia.
Conclusions
There has been much recent progress in the evolution standard procedures for IONM during thyroid and parathyroid surgeries in line with the recognition that neural visual integrity does not equate to functional viability and with an increasing focus on optimizing outcomes in neck endocrine surgical procedures. Standardization and organizational support have been instrumental in promoting the use of IONM and in increasing the number of publications that report intraoperative EMG data with their meaningful clinical interpretations. These trends are likely to continue and, as evolution of the equipment for neuromonitoring continues and our understanding of laryngeal neuroanatomy expands, the options for IONM of the RLN and EBSLN will continue to advance.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcin Barczyński, Hui Sun and Xiaoli Liu) for the series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.12.01). The series “The Protection and Monitoring of Superior and Recurrent Laryngeal Nerve in Thyroid and Parathyroid Surgery” was commissioned by the editorial office without any funding or sponsorship. Gregory W. Randolph serves as an unpaid editorial board member of Annals of Thyroid from Apr 2017 to Mar 2019. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jeannon JP, Orabi AA, Bruch GA, et al. Diagnosis of recurrent laryngeal nerve palsy after thyroidectomy: a systematic review. Int J Clin Pract 2009;63:624-9. [Crossref] [PubMed]
- Francis DO, Pearce EC, Ni S, et al. Epidemiology of Vocal Fold Paralyses after Total Thyroidectomy for Well-Differentiated Thyroid Cancer in a Medicare Population. Otolaryngol Head Neck Surg 2014;150:548-57. [Crossref] [PubMed]
- Shedd DP, Durham C. electrical identification of the recurrent laryngeal nerve. I. Response of the canine larynx to electrical stimulation of the recurrent laryngeal nerve. Ann Surg 1966;163:47-50. [Crossref] [PubMed]
- Riddell V. Thyroidectomy: prevention of bilateral recurrent nerve palsy. Results of identification of the nerve over 23 consecutive years (1946-69) with a description of an additional safety measure. Br J Surg 1970;57:1-11. [Crossref] [PubMed]
- Gavilán J, Gavilan C. Recurrent laryngeal nerve. Identification during thyroid and parathyroid surgery. Archives of otolaryngology Head and Neck surgery 1986;112:1286-8. [Crossref] [PubMed]
- Macias AA, Eappen S, Malikin I, et al. Successful intraoperative electrophysiologic monitoring of the recurrent laryngeal nerve, a multidisciplinary approach: The Massachusetts Eye and Ear Infirmary monitoring collaborative protocol with experience in over 3000 cases. Head Neck 2016;38:1487-94. [Crossref] [PubMed]
- Horne SK, Gal TJ, Brennan JA. Prevalence and patterns of intraoperative nerve monitoring for thyroidectomy. Otolaryngol Head Neck Surg 2007;136:952-6. [Crossref] [PubMed]
- Sturgeon C, Sturgeon T, Angelos P. Neuromonitoring in thyroid surgery: attitudes, usage patterns, and predictors of use among endocrine surgeons. World J Surg 2009;33:417-25. [Crossref] [PubMed]
- Ho Y, Carr MM, Goldenberg D. Trends in intraoperative neural monitoring for thyroid and parathyroid surgery amongst otolaryngologists and general surgeons. Eur Arch Otorhinolaryngol 2013;270:2525-30. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Loss of the nerve monitoring signal during bilateral thyroid surgery. Br J Surg 2012;99:1089-95. [Crossref] [PubMed]
- Mihai R, Chadwick D on behalf of BAETS, 2013 Annual Meeting, Rome.
- Hopkins C, Khemani S, Terry RM, et al. How we do it: nerve monitoring in ENT surgery: current UK practice. Clin Otolaryngol 2005;30:195-8. [Crossref] [PubMed]
- Godballe C. Registry of Surgical results: organization and outcomes. In 34th Annual meeting of the European Thyroid Association. ETA Lisbon 2009.
- Musholt TJ, Clerici T, Dralle H, et al. German Association of Endocrine Surgeons practice guidelines for the surgi- cal treatment of benign thyroid disease. Langenbecks Arch Surg 2011;396:639-49. [Crossref] [PubMed]
- Dionigi G, Lombardi D, Lombardi CP, et al. Intraoperative neuromonitoring in thyroid surgery: a point prevalence survey on utilization, management, and documentation in Italy. Updates Surg 2014;66:269-76. [Crossref] [PubMed]
- Dralle H, Sekulla C, Haerting J, et al. Risk factors of paralysis and functional outcome after recurrent laryngeal nerve monitoring in thyroid surgery. Surgery 2004;136:1310-22. [Crossref] [PubMed]
- Barczyński M, Konturek A, Pragacz K, et al. Intraoperative nerve monitoring can reduce prevalence of recur- rent laryngeal nerve injury in thyroid reoperations: results of a ret-rospective cohort study. World J Surg 2014;38:599-606. [Crossref] [PubMed]
- Barczyński M, Konturek A, Cichoń S. Randomized clinical trial of visualization versus neuromonitoring of recurrent laryngeal nerves during thyroidectomy. Br J Surg 2009;96:240-6. [Crossref] [PubMed]
- Thomusch O, Sekulla C, Walls G, et al. Intraoperative neuromonitoring of surgery for benign goiter. Am J Surg 2002;183:673-8. [Crossref] [PubMed]
- Zheng S, Xu Z, Wei Y, et al. Effect of intraoperative neuromonitoring on recurrent laryngeal nerve palsy rates after thyroid surgery-a meta-analysis. J Formos Med Assoc 2013;112:463-72. [Crossref] [PubMed]
- Pisanu A, Porceddu G, Podda M, et al. Systematic review with meta-analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res 2014;188:152-61. [Crossref] [PubMed]
- Sanabria A, Ramirez A, Kowalski LP, et al. Neuromonitoring in thyroidectomy: a meta-analysis of effectiveness from randomized controlled trials. Eur Arch Otorhinolaryngol 2013;270:2175-89. [Crossref] [PubMed]
- Kamani D, Potenza AS, Cernea CR, et al. The nonrecurrent laryngeal nerve: anatomic and electrophysiologic algorithm for reliable identification. Laryngoscope 2015;125:503-508. el. [Crossref] [PubMed]
- Dralle H, Sekulla C, Lorenz K, et al. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg 2008;32:1358-66. [Crossref] [PubMed]
- Chandrasekhar SS, Randolph GW, Seidman MD, et al. American Academy of Otolaryngology Head and Neck Surgery Clinical Practice Guidelines: improving voice outcomes after thyroid surgery. Otolaryngol Head Neck Surg 2013;148:S1-S37. [Crossref] [PubMed]
- Scharpf J, Tuttle M, Wong R, et al. Comprehensive management of recurrent thyroid cancer: An American Head and Neck Society consensus statement: AHNS consensus statement. Head Neck 2016;38:1862-9. [Crossref] [PubMed]
- Agrawal N, Evasovich MR. Indications and extent of central neck dissection for papillary thyroid cancer: An American Head and Neck Society Consensus Statement. Head Neck 2017;39:1269-79. [Crossref] [PubMed]
- Shindo ML, Caruana SM, Kandil E, et al. Management of invasive well-differentiated thyroid cancer: an American Head and Neck Society consensus statement. AHNS consensus statement. Head Neck 2014;36:1379-90. [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Terris DJ, Snyder S, Carneiro-Pla D, et al. American Thyroid Association Statement on outpatient thyroidectomy. Thyroid 2013;23:1193-202. [Crossref] [PubMed]
- Chen AY, Bernet VJ, Carty SE, et al. American thyroid association statement on optimal surgical management of goiter. Thyroid 2014;24:181-9. [Crossref] [PubMed]
- Randolph GW, Dralle H, Abdullah HInternational Intraoperative Monitoring Study Group, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope 2011;121:S1-16. [Crossref] [PubMed]
- Barczyński M, Randolph GW, Cernea CR, et al. External branch of the superior laryngeal nerve monitoring during thyroid and parathyroid surgery: International Neural Monitoring Study Group standards guideline statement. Laryngoscope 2013;123:S1-14. [Crossref] [PubMed]
- Potenza AS, Phelan EA, Cernea CR, et al. Normative intra-operative electrophysiologic waveform analysis of superior laryngeal nerve external branch and recurrent laryngeal nerve in patients undergoing thyroid surgery. World J Surg 2013;37:2336-42. [Crossref] [PubMed]
- Sritharan N, Chase M, Kamani D, et al. The vagus nerve, recurrent laryngeal nerve, and external branch of the superior laryngeal nerve have unique latencies allowing for intraoperative documentation of intact neural function during thyroid surgery. Laryngoscope 2015;125:E84-E89. [Crossref] [PubMed]
- Phelan E, Potenza A, Slough C, et al. Recurrent laryngeal nerve monitoring during thyroid surgery: normative vagal and recurrent laryngeal nerve electrophysiological data. Otolaryngol Head Neck Surg 2012;147:640-6. [Crossref] [PubMed]
- Caragacianu D, Kamani D, Randolph GW. Intraoperative monitoring: normative range associated with normal postoperative glottic function. Laryngoscope 2013;123:3026-31. [Crossref] [PubMed]
- Lamadé W, Meyding-Lamadé U, Buchhold C, et al. First continuous nerve monitoring in thyroid gland surgery. Chirurg 2000;71:551-7. [PubMed]
- Phelan E, Schneider R, Lorenz K, et al. Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 2014;124:1498-505. [Crossref] [PubMed]
- Schneider R, Randolph GW, Sekulla C, et al. Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 2013;35:1591-8. [Crossref] [PubMed]
- Cernea CR, Nishio S, Hojaij FC. Identification of the external branch of the superior laryngeal nerve (EBSLN) in large goiters. Am J Otolaryngol 1995;16:307-11. [Crossref] [PubMed]
- Kierner AC, Aigner M, Burian M. The external branch of the superior laryngeal nerve: its topographical anatomy as related to surgery of the neck. Arch Otolaryngol Head Neck Surg 1998;124:301-3. [Crossref] [PubMed]
- Selvan B, Babu S, Paul MJ, et al. Mapping the compound muscle action potentials of cricothyroid muscle using electromyography in thyroid operations: a novel method to clinically type the external branch of the superior laryngeal nerve. Ann Surg 2009;250:293-300. [Crossref] [PubMed]
- Lennquist S, Cahlin C, Smeda S. The superior laryngeal nerve in thyroid surgery. Surgery 1987;102:999-1008. [PubMed]
- Cernea CR, Ferraz AR, Nishio S, et al. Surgical anatomy of the external branch of the superior laryngeal nerve. Head Neck 1992;14:380-3. [Crossref] [PubMed]
- Kark AE, Kissin MW, Auerbach R, et al. Voice changes after thyroidectomy: role of the external laryngeal nerve. Br Med J (Clin Res Ed) 1984;289:1412-5. [Crossref] [PubMed]
- Lekacos NL, Miligos ND, Tzardis PJ, et al. The superior laryngeal nerve in thyroidectomy. Am Surg 1987;53:610-2. [PubMed]
- Teitelbaum BJ, Wenig BL. Superior laryngeal nerve injury from thyroid surgery. Head Neck 1995;17:36-40. [Crossref] [PubMed]
- Darr EA, Tufano RP, Ozdemir S, et al. Superior laryngeal nerve quantitative intraoperative monitoring is possible in all thyroid surgeries. Laryngoscope 2014;124:1035-41. [Crossref] [PubMed]
- Kochilas X, Bibas A, Xenellis J, et al. Surgical anatomy of the external branch of the superior laryngeal nerve and its clinical significance in head and neck surgery. Clin Anat 2008;21:99-105. [Crossref] [PubMed]
- Liddy W, Barber SR, Cinquepalmi M, et al. The electrophysiology of thyroid surgery: electrophysiologic and muscular responses with stimulation of the vagus nerve, recurrent laryngeal nerve, and external branch of the superior laryngeal nerve. Laryngoscope 2017;127:764-71. [Crossref] [PubMed]
Cite this article as: Sinclair CF, Kamani D, Randolph GW. The evolution and progress of standard procedures for intraoperative nerve monitoring. Ann Thyroid 2019;4:1.