Preconception counseling for thyroid disorders
Introduction
Transient changes in thyroid function occur during pregnancy. An elementary review of these changes with the patient and her partner during the preconception visit is prudent for understanding the basis for clinical recommendations and the importance of compliance with the treatment plan. Due to gestational hormonal changes and the fetal requirements for thyroid hormone in the first half of pregnancy, significant reversible changes in maternal thyroid physiology are noted. Elevated serum human chorionic gonadotropin concentrations in early pregnancy result in increased thyroid hormone production and a suppression of serum TSH concentrations by 10 weeks’ gestation leading to gestational thyrotoxicosis (1). This is a normal physiologic response and in the absence of other signs and symptoms of thyroid disease does not mandate further surveillance or treatment (2).
The fetal thyroid gland starts synthesizing thyroid hormone after 12 weeks and is not fully functional until 18 to 20 weeks’ gestation. Hence, for the first half of pregnancy, the fetus is dependent on the maternal supply of thyroxine (3). To ensure an adequate maternal and fetal supply, patients should understand that close monitoring of thyroid function and compliance with medication regimens is crucial in this time period.
Normal thyroid function
The physiological changes in thyroid function in pregnancy necessitate population-based and trimester-specific reference ranges for serum TSH and free T4 (FT4). However, if these data are not available, the American Thyroid Association guidelines recommend that between week 7 and 12, the lower limit of TSH be reduced by 0.4 mU/L and the upper limit be reduced by 0.5 mU/L. For the average patient, this translates into an upper limit for TSH of 4.0 mU/L. In the 2nd and 3rd trimesters, non-pregnancy TSH reference ranges may be used (4).
The upper reference range for total T4 should be increased by 5% for every week past 7 weeks’ gestation. By week 16, the upper reference range for total T4 is approximately 1.5-fold higher than in non-pregnant women. FT4 measurements by automated immunoassays may be altered due to the increase in thyroid-binding globulin. The FT4 index, calculated by measuring total T4 and T3 uptake appears to provide a more reliable FT4 estimate in pregnancy (5,6). The different reference ranges for thyroid tests in pregnancy can be confusing for patients and providers. General patient awareness of pregnancy reference ranges will allow for correct interpretation of thyroid test results as most laboratories will only report non-pregnancy ranges.
Iodine requirement
During pregnancy, iodine requirement almost doubles due to increased thyroid hormone production, rise in urinary iodine clearance and fetal iodine requirements. To achieve a daily total iodine intake of 250 µg, it has been recommended that women who are planning pregnancy or are pregnant or breastfeeding take iodine supplementation of 150 µg daily (4).
Hypothyroidism
Women with hypothyroidism should be counseled regarding the importance of optimizing their thyroid function parameters in the preconception period given the essential role of thyroid hormone in fetal brain development and cognitive functioning of the offspring. Routine preconception screening for thyroid disease is not recommended in healthy women. In pregnancy, an aggressive case-finding approach is supported. Risk factors include advanced maternal age, a personal or family history of thyroid or autoimmune disorders, living in an area of iodine deficiency or a personal history of infertility, miscarriage or preterm delivery (7).
During the preconception period, clinically hypothyroid women not previously on treatment should be started on levothyroxine at 1–2 µg/kg daily or approximately 100 µg/day (8). Women with a history of thyroid ablation or thyroidectomy may require higher doses. Prior to conception in a hypothyroid woman, if TSH is above 1 mU/L, the levothyroxine dosage should be adjusted by 25–50 µg increments and thyroid function monitored until TSH levels are between 0.5–2.5 mU/L. Additionally, women should be aware of the increased demand for levothyroxine during pregnancy and counseled to contact their endocrinologist or obstetrician once pregnancy is suspected or confirmed. Upon confirmation of pregnancy, the dose of levothyroxine can be increased by approximately 25–30% or the patient can take 2 additional tablets per week of the current levothyroxine dose (9,10). In this scenario, thyroid function tests should be obtained as soon as possible and the levothyroxine dosage adjusted accordingly. Waiting until the first prenatal visit to obtain labs may delay treatment as many women do not start prenatal care until 8 to 10 weeks of pregnancy. Since the majority of treated pregnant women will require further increases in levothyroxine dosing, TSH levels should be monitored every 6 weeks until the middle of pregnancy, or at least once every trimester if well controlled (11,12).
A discussion regarding the risk of adverse outcomes in pregnancy should include the increased risk of miscarriage, premature birth, low birth weight, placental abruption, gestational hypertension and stillbirth (13,14). It is important to emphasize that adequate thyroid hormone supplementation has been shown to improve pregnancy outcomes in clinical hypothyroidism (14).
Women should also be counseled that certain drugs interfere with levothyroxine absorption or metabolism including iron sulfate, aluminum hydroxide antacids, phenytoin and carbamazepine (8). It is best not to take levothyroxine at the same time as these medications. Furthermore, women taking levothyroxine regularly do not require additional iodine supplementation in pregnancy (4).
In women with hypothyroidism who are well controlled and have otherwise uncomplicated pregnancies, serial ultrasound monitoring of the fetus, antepartum testing or early delivery is not indicated unless other co-morbidities are present.
Hyperthyroidism
Most women with hyperthyroidism presenting for preconception counseling will have a confirmed diagnosis of Graves’ disease. Other causes of hyperthyroidism include toxic nodular goiter, toxic adenoma or thyroiditis. Women should be counseled that pregnancy should be postponed and adequate contraception used until a euthyroid state is achieved. This can be ascertained by at least two normal thyroid function tests 4 weeks apart in the absence of a change in therapy.
During the preconception visit, all valid treatment options should be presented and reviewed with the patient and her partner. Given that radioactive iodine treatment is contraindicated in pregnancy due to its effects on the fetal thyroid gland, women who are candidates for ablative therapy should undergo the procedure prior to pregnancy and use an effective method of contraception in the interim period. Pregnancy should be delayed until a stable euthyroid state has been reached. Surgery is also best undertaken prior to attempting pregnancy. Although thyroidectomy is not commonly performed in pregnancy, it can be undertaken, preferably in the 2nd trimester, if adherence to medical therapy is poor or if severe side effects from anti-thyroid drugs are a concern. Following definitive treatment, the patient’s thyroid status and TSH receptor antibody (TRAb) concentrations should be monitored. For patients stable on levothyroxine replacement therapy, pregnancy can be attempted (15).
Hyperthyroid women who are in a euthyroid state can avoid anti-thyroid medications in the first trimester of pregnancy. Under the guidance of a clinician, euthyroid women who have been well-controlled for at least 6 months, are on low doses of anti-thyroid drug and have favorable clinical factors may be good candidates for discontinuing thioamide therapy prior to or in early pregnancy (4). Initially, these women should be monitored closely with thyroid function testing every 1 to 2 weeks. At each assessment visit, the decision to continue expectant management or reinstate therapy should be re-evaluated. It is noteworthy that this ATA recommendation is from expert opinion and should be tested in a well-planned clinical trial.
Thyrotoxic women should be treated with thioamide drugs in the preconception period. Propylthiouracil (PTU) is the preferred agent in the first trimester but it has recently been associated with birth defects in 2–3% of exposed children including face and neck cysts and urinary tract abnormalities in males (16,17). Due to concern for hepatotoxicity with continued use of PTU in the pregnancy, switching to methimazole at 16 weeks should be considered (18). The recommendation to not use methimazole in the 1st trimester stems from the teratogenic potential of methimazole including methimazole embryopathy, abdominal wall defects and aplasia cutis (19). As the potential for adverse effects exists with all thioamide medications, the decision to switch medications should be based on each patient’s individual clinical situation. It should be noted that in pregnancy, FT4 should be maintained in the upper limit of normal to avoid overtreatment of the fetus. If beta adrenergic blocking agents such as propranolol are prescribed for controlling hyper-metabolic symptoms, they may be continued in pregnancy as needed. Physicians should review the teratogenicity profile of anti-thyroid medications and their potential side effects with patients and their partners. The preconception visit is an ideal time to discuss and weigh the benefits and risks of all treatment options.
A discussion regarding the risks of inadequately treated maternal thyrotoxicosis is also necessary. The incidence of maternal and fetal adverse outcomes is related to the control of hyperthyroidism. Complications such as congestive heart failure, thyroid storm, preterm birth, and preeclampsia can have grave consequences for the mother and fetus (20-22). Although thyroid storm is rare in pregnancy, it is a life-threatening emergency. Therefore, compliance with the medical treatment plan is of paramount importance. It should be emphasized that adequate treatment of hyperthyroidism in pregnancy has been shown to improve pregnancy outcomes (23).
Pregnancy is an immunosuppressive state. Hence, Graves’ disease may improve as the pregnancy progresses and TRAb titers may decrease. Discontinuation of anti-thyroid therapy will be possible in up to 30% of women with well-controlled disease by the 3rd trimester (24). Conversely, in the postpartum period, recurrence of disease may occur due to an increase in immunomodulatory activity. Close monitoring for recurrence and postpartum thyroiditis is indicated in the immediate and 6-week post-delivery periods.
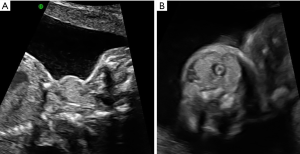
TRAb can cross the placenta and place the fetus at risk of goiter (Figure 1). Although in most cases the neonate will be euthyroid, in the setting of high maternal TRAb concentrations, there remains concern for immune-mediated neonatal hypothyroidism or hyperthyroidism in addition to delayed presentation of neonatal Graves’ disease. In women who have undergone a thyroidectomy or radioactive iodine ablative therapy prior to pregnancy, the risk of neonatal Graves’ disease is even higher due to the lack of suppressive thioamide treatment (25). Women with a history of Graves’ disease previously treated with ablation or surgery should have TRAb titers checked at the initial pregnancy visit. Repeat testing in the mid-trimester may be indicated if initial titers are elevated (3 times the upper limit of normal). In women taking anti-thyroid drugs, TRAb titers in early or mid-pregnancy are recommended and if elevated, repeat titers in the 3rd trimester should be performed to evaluate the need for intensive neonatal monitoring (4). Women with high TRAb titers should be counseled to communicate this finding to their obstetrical and pediatric teams at the time of delivery.
Close monitoring of pregnant women with sub-optimally controlled Graves’ disease should include serial ultrasounds for assessment of fetal size, fetal heart rate and fetal well-being given the increased risk of fetal growth restriction and stillbirth in this population. Women with elevated TRAb levels should be managed in consultation with a maternal fetal medicine sub-specialist to assess for evidence of fetal goiter at or after 20 weeks. Routine monitoring for evidence of fetal goiter in all pregnancies with Graves’ disease is not indicated. Thyroid function testing should be performed 2 to 4 weeks from the initiation of therapy or time of dosage change and be monitored every 4 to 6 weeks thereafter (15). Major guidelines consider subclinical hyperthyroidism to not pose risks to the mother or fetus during pregnancy (4,7).
Autoimmune thyroid disease
Thyroid peroxidase antibody (TPOAb) is the most frequently measured antibody in the setting of thyroid dysfunction and is present in up to 26% of the general population (5). It is an important marker of autoimmune thyroid disease. Risks associated with TPOAb positivity in pregnancy include miscarriage, preterm birth, placental abruption, fetal death and postpartum thyroiditis (26-31). Of note, the risk of adverse pregnancy outcomes is higher in women with both TPOAb positivity and elevated TSH levels but may be decreased by levothyroxine treatment (29,32). Routine TPOAb screening in women pursuing a pregnancy or with a confirmed pregnancy is not supported by national guidelines.
Subclinical hypothyroidism (elevated TSH and normal FT4) has been associated with pregnancy loss, premature delivery, preeclampsia and impaired cognitive function in the offspring in some but not all studies although many of these studies did not evaluate subjects for TPOAb positivity (7). Two large studies found that levothyroxine treatment of subclinically hypothyroid pregnant women had no beneficial effects on pregnancy outcomes (33), or cognitive function of offspring (33,34). However, both trials pooled TPOAb positive and negative women.
Given the paucity of data assessing the efficacy of interventions for improving long-term neurological development in the offspring, routine treatment for all pregnant women with subclinical hypothyroidism is not recommended (10). The American Thyroid Association recommends initiation of levothyroxine therapy at a starting dose of 50 µg for TPOAb positive pregnant women with TSH above the pregnancy specific range and for TPOAb negative pregnant women with TSH >10.0 mU/L. Additionally, they support treating TPOAb positive women with TSH between 2.5 mU/L and the upper limit of the pregnancy range and TPOAb negative women with TSH between the pregnancy reference range and 10.0 mU/L (4).This recommendation has been confirmed by a recent clinical trial in TPOAb negative pregnant women (35). TPOAb positive euthyroid women at high risk for developing hypothyroidism should be screened for TSH abnormalities during preconception counseling, at time of pregnancy confirmation and again in the 1st and 2nd trimesters (3,7).
Thyroid nodules
Thyroid nodules are found in 1% to 2% of reproductive aged women (36). For women presenting with a thyroid nodule in the preconception period, a clinical exam, complete history and physical, thyroid function testing and a thyroid ultrasound should be performed. If findings are suspicious for malignancy, final needle aspiration is indicated followed by surgery if nodules prove malignant. In women who have undergone definitive treatment, pregnancy should be delayed by at least 6 months to ensure a stable thyroid function. During pregnancy, definitive surgery can be performed for thyroid cancer in cases of rapid growth or lymph node involvement or may be deferred until the postpartum period. Thyroid hormone may be administered in pregnant women with active or prior thyroid cancer to suppress TSH levels (37). In cases where thyroid cancer is part of a hereditary familial malignancy syndrome, genetic counseling should be considered (10).
A suppressed TSH in the setting of a thyroid nodule should prompt evaluation with scintigraphy to evaluate nodule function. As scintigraphy is contraindicated in pregnancy, an autonomous thyroid nodule should be treated with ablative therapy prior to planning a pregnancy. Treatment of autonomous nodules with anti-thyroid drugs in pregnancy is acceptable but carries a higher risk for fetal hypothyroidism and goiter (2). Women with autonomous nodules should avoid iodine-containing prenatal vitamins.
Context of preconception counseling
Table 1 shows a summary of preconception counseling for thyroid disorders. Routine screening is not recommended for all women planning for pregnancy. Those with serum TSH above 4 mU/L should be treated with levothyroxine to achieve TSH <2.5 mU/L. TPOAb positive women with TSH between 2.5–4 mU/L may be treated or observed closely. Those with TSH <2.5 mU/L should be observed closely and serum TSH obtained after conception. Most hypothyroid patients should receive higher doses of levothyroxine. Hyperthyroid patients should be rendered euthyroid before conception and methimazole changed to propylthiouracil. In well-controlled low risk patients, one may consider discontinuation of anti-thyroid medication with close observation. Ablative treatment may be recommended if pregnancy planning can be postponed.
Table 1
| Status of women | Recommendation |
|---|---|
| All women | Targeted TSH testing in women at high risk for hypothyroidism |
| TPOAb positivity | Obtain TSH; treatment with levothyroxine for all with TSH >4.0 mU/L and perhaps TSH 2.5–4 mU/L. Close observation of those with TSH <2.5 mU/L and TSH testing after conception |
| Hypothyroidism | Increase levothyroxine dose by 25–30% (or add 2 tables of 100 µg per week) in all with TSH >1 mU/L |
| Hyperthyroidism | Adjust treatment to obtain euthyroidism |
| Change methimazole to propylthiouracil or discontinue antithyroid medication in well controlled patients. Consider ablative treatments in some patients |
Conclusions
Preconception counseling plays a key role in preparing women with thyroid dysfunction for a successful pregnancy. Improving disease activity and modifying treatment regimens to achieve optimal outcomes with the correct balance of benefit versus risk is the ultimate goal. Contraceptive management is an important component of preconception counseling as unintended pregnancies in the setting of poorly controlled thyroid function can result in increased maternal and fetal morbidity and mortality. Multidisciplinary care of patients with thyroid disease that include an endocrinologist, obstetrician, maternal fetal medicine sub-specialist and neonatologist will help optimize the health of the mother and her fetus during pregnancy and beyond.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Fereidoun Azizi) for the series “Thyroid and Pregnancy” published in Annals of Thyroid. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.07.02). The series “Thyroid and Pregnancy” was commissioned by the editorial office without any funding or sponsorship. The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the manuscript and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ballabio M, Poshychinda M, Ekins RP. Pregnancy-induced changes in thyroid function: role or human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab 1991;73:824-31. [Crossref] [PubMed]
- Patil-Sisodia K, Mestman JH. Graves hyperthyroidism and pregnancy: a clinical update. Endocr Pract 2010;16:118-29. [Crossref] [PubMed]
- Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med 1994;331:1072-8. [Crossref] [PubMed]
- Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017;27:315-89. [Crossref] [PubMed]
- Lee RH, Spencer CA, Mestman JH, et al. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 2009;200:260.e1-6. [Crossref] [PubMed]
- Azizi F, Mehran L, Amouzegar A, et al. Establishment of the trimester-specificreference range for free thyroxine index. Thyroid 2013;23:354-9. [Crossref] [PubMed]
- De Groot L, Abalovich M, Alexander EK, et al. Management of thyroiddysfunction during pregnancy and postpartum: an EndocrineSociety clinical practice guideline. J Clin Endocrinol Metab 2012;97:2543-65. [Crossref] [PubMed]
- Casey BM, Leveno KJ. Thyroid disease in pregnancy. Obstet Gynecol 2006;108:1283-92. [Crossref] [PubMed]
- Yassa L, Marqusee E, Fawcett R, et al. Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab 2010;95:3234-41. [Crossref] [PubMed]
- Practice Bulletin No. 148: Thyroid disease in pregnancy. Obstet Gynecol 2015;125:996-1005. [Crossref] [PubMed]
- Mandel SJ, Larsen PR, Seely EW, et al. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med 1990;323:91-6. [Crossref] [PubMed]
- Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004;351:241-9. [Crossref] [PubMed]
- Taylor PN, Minassian C, Rehman A, et al. TSH levels and risk of miscarriage in women on long-term levothyroxine: a community-based study. J Clin Endocrinol Metab 2014;99:3895-902. [Crossref] [PubMed]
- Abalovich M, Gutierrez S, Alcaraz G, et al. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid 2002;12:63-8. [Crossref] [PubMed]
- Korevaar TIM, Medici M, Visser TJ, et al. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 2017;13:610-22. [Crossref] [PubMed]
- Bahn RS, Burch HS, Cooper DS, et al. The role of propylthiouracil in the management of Grave’s disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid 2009;19:673-4. [Crossref] [PubMed]
- Andersen SL, Olsen J, Wu CS, et al. Birth defects after early pregnancy use of antithyroid drugs: a Danish nationwide study. J Clin Endocrinol Metab 2013;98:4373-81. [Crossref] [PubMed]
- Bahn RS, Burch HS, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract 2011;17:456-520. [Crossref] [PubMed]
- Yoshihara A, Noh J, Yamaguchi T, et al. Treatment of Graves’ disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab 2012;97:2396-403. [Crossref] [PubMed]
- Davis LE, Lucas MJ, Hankins GD, et al. Thyrotoxicosis complicating pregnancy. Am J Obstet Gynecol 1989;160:63-70. [Crossref] [PubMed]
- Millar LK, Wing DA, Leung AS, et al. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol 1994;84:946-9. [PubMed]
- Sheffield JS, Cunningham FG. Thyrotoxicosis and heart failure that complicate pregnancy. Am J Obstet Gynecol 2004;190:211-7. [Crossref] [PubMed]
- Kriplani A, Buckshee K, Bhargava VL, et al. Maternal and perinatal outcome in thyrotoxicosis complicating pregnancy. Eur J Obstet Gynecol Reprod Biol 1994;54:159-63. [Crossref] [PubMed]
- Hamburger JI. Diagnosis and management of Grave’s disease in pregnancy. Thyroid 1992;2:219-24. [Crossref] [PubMed]
- Laurberg P, Nygaard B, Glinoer D, et al. Guidelines for TSH-receptor antibody measurements in pregnancy: results of an evidence-based symposium organized by the European Thyroid Association. Eur J Endocrinol 1998;139:584-6. [Crossref] [PubMed]
- Stagnaro-Green A, Roman SH, Cobin RH, et al. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. JAMA 1990;264:1422-5. [Crossref] [PubMed]
- Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol 2004;150:751-5. [Crossref] [PubMed]
- Glinoer D, Soto MF, Bourdoux P, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussion. J Clin Endocrinol Metab 1991;73:421-7. [Crossref] [PubMed]
- Negro R, Schwartz A, Gismondi R, et al. Thyroid antibody positivity in the first trimester is associated with negative pregnancy outcomes. J Clin Endocrinol Metab 2011;96:E920-4. [Crossref] [PubMed]
- Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab 2013;98:4382-90. [Crossref] [PubMed]
- Thangaratinam S, Tan A, Knox E, et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342:d2616. [Crossref] [PubMed]
- Nazarpour S, Ramezani Tehrani F, Simbar M, et al. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol 2017;176:253-65. [Crossref] [PubMed]
- Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med 2017;376:815-25. [Crossref] [PubMed]
- Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med 2012;366:493-501. [Crossref] [PubMed]
- Nazarpour S, Ramezani Tehrani F, Simbar M, et al. Effects of Levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J Clin Endocrinol Metab 2018;103:926-35. [Crossref] [PubMed]
- Fitzpatrick DL, Russell MA. Diagnosis and management of thyroid disease in pregnancy. Obstet Gynecol Clin North Am 2010;37:173-93. [Crossref] [PubMed]
- Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am 2012;96:329-49. [Crossref] [PubMed]
Cite this article as: Aghajanian P. Preconception counseling for thyroid disorders. Ann Thyroid 2018;3:16.