Medullary thyroid cancer: strategy, pitfalls and technical aspects with emphasis on remedial surgery
Introduction
Usually, medullary thyroid cancer (MTC) shows to be highly aggressive compared with the differentiated thyroid one and when it compromises young patients’ higher rates of recurrence and mortality are present (1,2).
MTC is associated with over 13% of all thyroid cancers related mortality. In MTC, early diagnosis with calcitonin (Ctn) screening is particularly important, as MTC can be cured only in the early stages. There is a possibility of hereditary disease with close genotype-phenotype correlation, which is of great importance for the prophylaxis of MTC disease (3,4). Furthermore, there are excellent tumor markers with Ctn and the carcinoembryonic antigen (CEA), which in the case of persistent disease are to be used primarily for oncological imaging in order to assess tumor biology (5).
The MTC disease is biologically heterogeneous, so the assessment of the course requires great experience. Although 20% of the MTCs cases revealed to be hereditary, the majority of the rest are sporadic; hereditary cases are likely to be found in multiple endocrine neoplasia (MEN) 2A and MEN 2B, as part of familial MTC based upon a peculiar RET proto-oncogene germline mutation. The incidence of MTC increased overall and across all stages of disease (2).
In this review article, diagnostic and therapeutic principles of MTC as neuroendocrine thyroid carcinoma are presented with particular emphasis on remedial surgery (3).
Pathology
MTC is an infrequent, Ctn producing neuroendocrine tumor and initiates from the parafollicular C cells of the thyroid gland. About 3–5% of all thyroid carcinomas are MTC (5) (Figure 1). MTCs are neuroendocrine tumors from Ctn-producing C-cells of the thyroid, accounting for approximately 0.1% of all thyroid epithelial cells (6,7).
MTC are therefore also referred to as C-cell carcinomas (5,6). The identification of precursor or dysplastic lesions in the thyroid is difficult. C cells are mainly located in the dorsolateral portion of the thyroid lobes, while the poles of the lobes and the isthmus are C-cell-free (7). Several extensive studies were carried out on the C-cell pathology, and the pre-neoplastic nature concerned with hyperplasia in the inception of familial MTCs is now well known (1-8). Yet it is still a challenge making a net distinction between neoplastic and physiologic/reactive C-cell hyperplasia (CCH). Opposed to C-cell, it is less evidently established the existence of a precursor lesion of the follicular cell derived tumors therefore a dysplastic or preneoplastic follicular lesion has not been well-marked (7).
Several genetic and epigenetic alterations are collaterally responsible for medullary thyroid carcinogenesis. From the genetic perspective, RET, HRAS, and KRAS are the most important genes that are characterized in MTC (7,8). From the epigenetic perspective, RAS-association domain family member 1A, telomerase reverse transcriptase promoter methylations, overexpression of histone methyltransferases, EZH2 and SMYD3, and wide-ranging increase and decrease in non-coding RNAs can be responsible for medullary thyroid carcinogenesis (7,9).
The MTC can occur in a wide variety of histological forms, therefore an MTC should be excluded immunohistochemically with the slightest suspicion and with all thyroid tumors with unusual morphology. The purpose of this is the determination of Ctn, ideally together with chromogranin A and CEA, as well as thyroglobulin (9).
Rarities are an “atypical MTC” with lack of Ctn expression in immunohistochemistry and a “non-secretory MTC” without serological Ctn elevation (2,7). The MTC distinguishes CCH, which is defined microscopically by at least 50 C-cells/field of view at the highest C-cell density at ×100 magnification. It occurs as a neoplastic CCH in hereditary MTC [CCH carcinoma sequence (6-8)]. A non-neoplastic CCH, however, is found in sporadic MTC and as a cause of Ctn elevation even in healthy or otherwise pathologically altered thyroid gland (7,9-12).
Sporadic and hereditary MTC
The sporadic form is more common (80%). Sporadic form etiology has not been defined clearly so far. Diagnosis is currently made with the routine use of serum Ctn measurements to screen patients with nodular thyroid disease. About 20% of MTCs are hereditary and are due to a germline mutation of the RET proto-oncogene (8,13). The aggressiveness of hereditary MTC and the presentation of other endocrine diseases are related to specific RET mutations.
The RET proto-oncogene encrypts a tyrosine kinase receptor for the members of the glial cell-line derived neurotrophic factor (GDNF) family of the signaling extracellular molecules (5). The mutations cause of RET loss of function are related to the development of different types of human cancer, which include as follows: MTC, type 2A and type 2B MENs, pheochromocytoma and parathyroid hyperplasia (1,7,8).
Since 1993, when RET proto-oncogene mutations were originally reported in MEN2, a growing number of about 46 different RET-mutations cause of disease have been reported (1). RET mutation carriers, MTC and other endocrine diseases need be diagnosed and treated early on account of their RET genotypes (1). They are autosomal dominant inherited as isolated familial MTC (FMTC) or as part of a MEN 2A or 2B (1).
In 98% of all MEN2 patients, the detection of a RET germline mutation is successful. This is almost equivalent to an almost 100% probability of MTC disease. For treatment stratification in hereditary MTC, close RET genotype-phenotype correlation is important, which allows predictions of MTC manifestation age as well as the likelihood of further MEN2 tumors (14,15). The recommendations for thyroidectomy are no longer only genotype-dependent but are also based on the basal Ctn value, the thyroidectomy can be done later (1-4).
The most aggressive course is observed in MEN 2B disease with mutations in RET codon 918. Here, the MTC already manifested in the first year of life. Early thyroidectomy is therefore essential. However, the vast majority of MTC occurs sporadically (70–80%). The average age of patients diagnosed is 45–50 years. Also, in sporadic MTC there are 40–60% mutations in the RET proto-oncogene, especially a somatic mutation in codon 918 (MEN 2B locus), more rarely are somatic RAS mutations (11).
Morphologically, hereditary MTCs differ from their sporadic counterparts by bilateral and/or multifocal appearance as well as by the presence of a simultaneous CCH. Any patient with initial diagnosis of MTC must have a human genetic examination for a RET germline mutation (15-17). In newly diagnosed hereditary MTC, screening of all first-degree relatives for the presence of a RET germline mutation is recommended (1-4,18,19). RET genetic screening of those patients with seemingly sporadic MTC is one of the most important tool to be used in preclinical diagnosis to work out an early treatment of the unaware and unsuspected family members who are affected by the disease; that anyhow would allow to identify an important percentage of concealed FMTC cases, thus helping the achievement of safe clinical benefits of RET screening in patients with clear sporadic MTC and that also to find out and identify the hereditary nature of the disease, discovering family members unaware of such condition but harboring the identical mutations of the germline, i.e., gene carriers.
Finally, MTC has varying clinical course. Survival in sporadic as well as hereditary MTC is critically dependent on tumor spread at the time of diagnosis. Larger tumors, cervical lymph node (LN) invasion, distant metastasis at follow-up, postoperative high Ctn levels are independently associated with 10-year disease progression. Clinically, cervical LN metastases are present in at least 50% of patients at the time of first diagnosis of MTC—this applies to sporadic as well as hereditary de novo carcinomas. Remote metastases in the liver, lung, bone or central nervous system (CNS) are found in 10–20% of patients (2). As the MTC often grows slowly, patients with distant metastases can survive for decades. The 10-year survival rate for distant metastasis is 25–50%
Preoperative diagnosis
Ctn and proCtn
Thyroid surgery must always be preceded by Ctn determination. Early diagnosis in MTC is particularly important. Ctn is the most sensitive and specific screening parameter available, and its measurement is decisive to obtain an early diagnosis and during the follow-up period of patients with MTC. Ctn is a 32-amino acid linear polypeptide hormone secreted primarily by the parafollicular cells (3). Its action results in the reduction of blood calcium levels, thus opposing the effects elicited by the parathyroid hormone (PTH) (4).
Should the evaluation of aroused Ctn levels be necessary, a provocative test can be performed, given the recent introduction in clinical practice of the high-dose calcium test. Most endocrine societies recommend Ctn determination in the context of clarification of an euthyroid thyroid nodule, prior to thyroid surgery (3,11). The peculiarities of Ctn determination should be taken into account when assessing its values, i.e., assays and gender-specific reference ranges, false-positive findings in renal insufficiency, the influence of proton pump inhibitors and currently unclear cut-off values in calcium stimulation.
Authors have investigated the role of basal versus calcium-stimulated Ctn in the preoperative diagnosis of MTC (12,13,16,17). In a specific study, both basal Ctn (bCtn) and stimulated Ctn (sCtn) values before thyroidectomy of 91 patients were compared with histological results; bCtn and sCtn levels showed a similar accuracy in the preoperative diagnosis of MTC, and the stimulating test was assessed as faisable (17). Moreover, gender-specific bCtn and sCtn cutoffs for detection of C-cell hyperplasia and/or MTC have been well-defined (17).
Machens et al. confirmed the relationship between the incremental serum computed tomography (CT) level before surgical second-look and the number of metastatic LN harvested previously (19); thus, stated that increased serum CT values (inferior or equal to 1,000 pg/mL) before reoperation and the amount of 5 or fewer LN metastases removed at first surgical procedure, suggest a systematic a LN dissection for persistent MTC (1,3,19).
Molecular diagnostics
Molecular diagnostics for MTC detection and genotype-based treatment algorithms are essential. Any preoperative suspicion of MTC (basal Ctn levels >26 pg/mL in females and >68 pg/mL in males), a molecular diagnosis of a RET germline mutation should be made prior to surgery on 2–5 mL of EDTA blood. In the presence of a hereditary MTC preoperative pheochromocytoma and primary hyperparathyroidism need to be excluded (4).
Staging
Staging is the process of neoplasm classification according to the extent of a tumor and how far it has spread. The stage of a cancer is one of the most important factors to choose the better option of treatment and predict the chances for a good adequate cure. At values <20 pg/mL (normal range <10 pg/mL) it can be assumed that LN metastases are not present, at values ≤200 pg/mL LN metastases in the central and ipsilateral, at values >200 pg/mL can also be present in the contralateral compartment (19-25).
Preoperative MTC staging involves multi-modal imaging with ultrasound (US), CT of the neck or thorax, 3-phase CT or magnetic resonance imaging (MRI) of the liver, MRI of the spine and bone scintigraphy for the exclusion or detection of distant metastases (26).
Treatment
Surgeon should be aware of the importance of preoperative diagnosis of MTC and Ctn-based treatment planning. Most common issues in MTC treatment are: (I) extent of initial surgery based on tumor size and preoperative Ctn and CEA levels; (II) how to identify loco regional disease (surgical curable) vs. distant spread [CT of the neck and superior mediastinum and positron emission tomography (PET) indicated in cases of serum Ctn >100 pg/mL before initial surgery]; (III) MTC is not sensitive to RAI; (IV) hormone suppression is ineffective; (V) recurrence which needs re-interventions.
Surgical therapy
The surgical procedure is measured by Ctn-based tumor values. The initial surgical resection is critical for obtaining biochemical and anatomic control. The surgical treatment of MTC is influenced by several factors: (I) in 90% of the patients with hereditary MTC and in 20% with sporadic form, the disease is multifocal and bilateral; (II) more than 70% of patients with nodal metastases present with palpable disease; (III) the possibility to rate levels of postoperative Ctn allows the assessment of the surgical extirpation adequacy; (IV) the prognosis of MTC depends on the stage and completeness of resection. Surgical therapy should be performed at a center equipped with a surgeon experienced in MTC therapy.
Adherence to American Thyroid Association (ATA) guidelines resulted in fewer cancer-related reoperations (P=0.027), more LNs examined (P=0.016) and more biochemical cure (P=0.038) (27-34). In patients with sporadic MTC is recommend the first surgical resection including total thyroidectomy and central LN dissection; the extent of lateral compartment is guided and suggested by the clinical detection of metastatic lymphadenopathy (by means of physical examination or US) and by the elevation of preoperative serum bCtn and CEA levels as well.
In addition to total thyroidectomy, a compartmentalized central and ipsilateral-lateral LN dissection is recommended for preoperative basal Ctn levels >20–200 pg/mL (normal range <10 pg/mL) (2-4). The indication for dissection of the contralateral-lateral compartment with Ctn >200 pg/mL depends on the extent of the LN findings, the imaging evidence of LN metastases in the contralateral compartment and the operative risk of the individual patient (2-4). If distant metastases are already present, the operative target should be geared to the adequate reduction of the local tumor problem, depending on the extent of distant metastases (2-4).
Asymptomatic carriers of a RET germline mutation
Total thyroidectomy is obligatory in hereditary MTC due to the presence of the RET germline mutation in the C cells. For asymptomatic carriers of a RET germline mutation, without LN metastases, at a normal basal Ctn level (normal range <10 pg/mL), LN dissection can be dispensed (thus lower risk of hypoparathyroidism) (3,16,17).
The concept of prophylactic thyroidectomy in RET mutation carriers has significantly improved the prognosis of hereditary MTC disease. The timing of surgery depends on the type of RET germline mutation, the age of the patient and the basal Ctn level. The operation should be performed prior to the earliest MTC manifestation of the particular RET genotype described in the literature. In the most common RET germline mutation in codon 634, for example, prophylactic thyroidectomy is recommended at preschool age, at the latest when the basal Ctn level reaches the upper reference range. Surgery may also be performed at a later date if and until the basal Ctn level exceeds the upper limit (1,3,4).
Evaluation of patients following initial surgery foR MTC
Postoperative period of patients treated for MTC is important in order to evaluate the thyroidectomy efficacy, whether it may be considered curative. Immediately postoperative thyroid hormone replacement is initiated with levothyroxine. The dosage is weight-adapted (1.6–1.8 µg levothyroxine/kg).
Central elements of MTC after-care after first-line therapy are: (I) the clinical examination; (II) the determination of the tumor markers Ctn and CEA; (III) a high-resolution cervical sonography; (IV) the verification of levothyroxine substitution (target: thyroid stimulating hormone in the normal range); (V) the review of the therapy of postoperative hypoparathyroidism
The following criteria are in favor of a biochemical cure of the MTC: (I) no clinical evidence of MTC; (II) no evidence at US; (III) Ctn levels repeated below detection limit of Assay; (IV) CEA within normal range.
In the absence of evidence of persistent MTC disease following primary surgical therapy, follow-up visits with Ctn and CEA determination and US in the 6-month interval and then recommended annually. It is essential to continue to monitor the adequate replacement therapy with levothyroxine and possibly calcitriol/calcium in hypoparathyroidism (1,4,8).
Special features of MEN2 disease
About 50% of patients with MEN 2A/B develop pheochromocytomas, bilaterally and multicentrically. Up to 10% of MEN 2A patients develop primary hyperparathyroidism. Often there is no typical clinical symptom. The extrathyroid MEN2 manifestations also depend on the RET genotype. The prevalence and the time of onset of extra-thyroid MEN2 manifestations are genotype-dependent (4).
Persistent or recurrent MTC
The staging system
The AJCC staging system (8th edition, 2016) has been applied to patients with MTC. This includes: T3a, “tumor >4 cm, limited to thyroid; T3b, gross extrathyroidal extension invading only strap muscle (35). Age with poor prognosis is >55 years. It must be noted that the TNM classification, in patients with MTC, lacks important prognostic factors such as (I) pre- and postoperative serum Ctn levels (and CEA); (II) genetic markers, including specific mutations; and (III) hereditary associated malignancies (35).
Furthermore, the AJCC TNM classification for MTC categorizes LN metastases in accordance with nodal location, within (N1a) or without (N1b) the central neck compartment, regardless number of metastatic LNs and involved compartments (35). The AJCC staging system should be modified to include groups of LN metastases according to number of positive nodes (35-38).
Strategies for surveillance
Postoperative normalization of Ctn serum levels is associated with a favorable outcome, however, there has been controversy regarding how long it takes to reach its nadir (38,39). Some clinical investigators have proposed that a 3-month length post-operation represent the optimal period to achieve the nadir of serum Ctn levels. Conversely, considering its prolonged half-life, serum levels of CEA may take even longer to reach the fewest level (39).
Other investigators consider a basal or stimulated serum Ctn level at or below the limits as indicative of a curative thyroidectomy for MTC (39). Other authors consider a curative thyroidectomy when the postoperative sCtn value is less than 10 pg/mL (39).
In a study on 124 patients having total thyroidectomy for tumors other than MTC, 97% had no serum Ctn detectable (38). In those with detectable Ctn levels, there was either residual thyroid tissue or ectopic secretion of Ctn from a non-thyroid malignancy. These data indicate that postoperative serum Ctn levels should result undetectable after the complete removal of the thyroid tissue. In the same study 32 out of 68 patients with MTC treated by total thyroidectomy had undetectable bCtn and sCtn postoperative levels, therefore appeared cured. Of the remaining 36 patients, 22 had pathologically increased bCtn levels and 11 of them revealed a residual MTC tissue, identified postoperatively; progressive increasing of Ctn levels in 11 patients indicated a tumor recurrence (38).
In a study on 63 consecutive patients treated by total thyroidectomy for MTC, suggest that the degree of elevation of serum Ctn levels can be used as marker to evaluate the disease extent. As a matter of fact, the postoperative bCtn level was less than 10 pg/mL in 35 patients and their 3- and 5-year relapse-free survival rates were 94% and 90%, respectively, in opposition to 78% and 61% in patients with bCtn levels above 10 pg/mL (40). In patients with bCtn levels less than 150 pg/mL following thyroidectomy, persistent or recurrent disease is almost always confined to the cervical LNs (40).
Features of persistent or recurrent MTC
Little is known about what proportion of Patients with persistent or recurrent MTC undergo reoperation or the factors that influence the decision to proceed with remedial surgery. Ctn levels remain detectable in 40-66% of patients after initial surgery for MTC. There are two residual MTC categories: (I) group A: patients who had an appropriate initial surgical resection (e.g., at least a total thyroidectomy with central neck dissection); (II) group B: patients who had lesser initial surgical procedures. Previous surgical and pathological reports should be reviewed for the extent of LN dissection to allow the assessment of the therapy. Orientation values are about 10 LNs in the central compartment and 20 LNs each in the lateral compartments. Second intervention should always be performed by a surgeon experienced in thyroid carcinoma therapy.
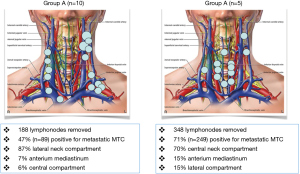
Tables 1,2 and Figure 2 summarize our recent experience on 15 consecutive patients who underwent remedial surgery for persistent or recurrent MTC. Patients who had lesser initial surgical procedures, warrant cervical re-exploration. Surgery includes complete removal of cervical and mediastinal lymphatic and fibrofatty tissue.
Table 1
| Characteristic | Group A (n=10) | Group B (n=5) |
|---|---|---|
| Type MTC | ||
| Sporadic | 7 | 4 |
| Familiar | 2 | 1 |
| MEN 2A | 1 | 0 |
| MEN 2B | 0 | 0 |
| Indications for reoperation | ||
| Hypercalcitoninemia | 6 | 4 |
| Symptomatic | 3 | 0 |
| Palliative | 1 | 1 |
| TNM Stage | ||
| I | 0 | 0 |
| II | 1 | 1 |
| III | 8 | 4 |
| IV | 1 | 0 |
| Distant metastases (after surgery) | 2 | 1 |
| Dead/alive | 1/10 | 1/4 |
MTC, medullary thyroid cancer; MEN, multiple endocrine neoplasia type.
Table 2
| Laboratory | Group A (n=10) | Group B (n=5) |
|---|---|---|
| Basal calcitonin level (ng/L) | 1.396–25.150 | 2.499–30.680 |
| After re-exploration | ||
| Undetectable | 1 | 0 |
| Reduced >50% | 6 | 4 |
| Reduced <50% | 3 | 1 |
MTC, medullary thyroid cancer.
If the tumor markers persistently increase, the possibility of a remedial surgical intervention with a curative objective should be examined, especially in the case of borderline or only moderate Ctn elevation (17).
Patients with residues of LN metastases after thyroidectomy are more likely to benefit from re-operation. In a study on 334 patients a second surgical procedure was performed for persistent MTC, as evidenced by elevated serum Ctn levels. A systematic central and lateral LN dissection attained biochemical cure in 44% of patients who had no metastases in LNs removed at prior surgery (36). Moreover, this report suggests that systematic LN dissection in patients who had an inadequate lymphadenectomy at first surgery is worthwhile, as long as the preoperative serum Ctn level is less than 1,000 pg/mL and five or fewer metastatic LNs were resected initially. Otherwise, the target of surgical treatment shifts to the maintenance of cervical local control (36).
The opposite thyroid lobe should be removed in patients with hereditary MTC because the likelihood that neoplasm will develop is 100%. In patients with sporadic MTC the incidence of bilateral MTC ranges from 0–9%. In a prospective study treated by hemithyroidectomy for sporadic MTC, 80% achieved a postoperative biochemical cure. Completion thyroidectomy is indicated in patient with RET germline mutation, a significant elevation of postoperative bCtn or sCtn levels, or imaging studies showing residual MTC. Metastases to regional nodes and large tumor size were adversely related to biochemical cure. During follow-up, a progressive increase in the bCtn level above 150 pg/mL should prompt additional imaging (37).
Reoperative procedure considerations
Reoperations entail an excess of surgical morbidity and may be avoidable. Efforts must be made to keep surgical morbidity to a minimum (Table 3). The risk of complications in reoperations for MTC may often be influenced by the disease progression as well as by preceding operative procedures and must be balanced with the likelihood of achieving biochemical cure and ultimately disease-free survival. Differences in patients’ primary surgical procedure, differences in patients’ primary disease stage at presentation, differences in location and number of compartments (central and/or lateral, unilateral vs. bilateral), and the number of reoperations performed are all likely to affect the ability to achieve a biochemical cure at the time of reoperation. Further research is needed in this field.
Table 3
| Considerations |
| Review prior operative notes |
| Preoperative laryngeal examination |
| Localization studies mandatory |
| Open conventional surgery: maximum exposure of the operative field |
| Dedicated surgical centers |
| Proper timing |
| “Back door” approach |
| Meticulous compartment-oriented dissection |
| Optical magnification |
| Nerve monitoring |
| Preservation of the inferior thyroid artery |
| Careful identification of the thoracic duct |
| Frozen section use |
| In situ preservation or autografting parathyroid glands |
Many complications occurred as a function of the number of LNs removed (19). In a study morbidity was associated with LN dissection as: (I) postoperative hypoparathyroidism (means of 58 and 69 dissected nodes for transient and permanent hypoparathyroidism vs. 49 dissected nodes; P=0.031); (II) postoperative hemorrhage (means of 75 vs. 51 dissected nodes; P=0.032); (III) postoperative wound infection (means of 130 vs. 51 dissected nodes; P=0.001); (IV) lymphatic fistula (means of 82 vs. 52 dissected nodes; P=0.045). No such dependency was observed for recurrent laryngeal nerve palsy (means of 53 and 67 dissected nodes for transient and permanent recurrent laryngeal nerve palsy vs. 52 dissected nodes; P=0.57).
Because of the relevant risk of associated with re-operative neck surgery, elevated postoperative Ctn levels should be combined with a careful metastatic evaluation by imaging studies, also for distant metastases, prior to considering re-do surgery. Furthermore, no randomized controlled trials have evaluated the long-term efficacy of reoperation in patients with recurrent MTC.
Palliation
If a surgical cure is no longer possible, there is a palliative situation. The term palliation should be used with caution in MTC, as the individual prognosis depends considerably on tumor burden and tumor biology. First, an assessment of the tumor burden is required. If there is no evidence of locoregional LN metastases, the probability of distant metastases increases from a basal Ctn level of 150 pg/mL. It can be detected using multimodal imaging, which includes an MRI or CT of the skull, a spiral or thin-layer CT of the thorax, a US of the neck and liver, MRI of the liver, MRI of the spine and pelvis, and skeletal scintigraphy.
Tumor biology is estimated by the doubling time of the tumor markers Ctn and CEA. In the first postoperative year, the tumor markers in persistent disease should be determined every 3 months. Even basally high CEA values indicate a poorly differentiated MTC and a less favorable course. With respect to both tumor markers, short doubling times <6 months indicate an unfavorable prognosis. In this case, regular staging with multimodal imaging is also recommended in the current ETA recommendations. Doubling times of >2 years, on the other hand, indicate a favorable prognosis.
Patients with >2 years of Ctn doubling time and lack of morphological tumor imaging have a very good prognosis, so MTC-specific follow-up can focus on tumor marker detection and Hals sonography. Restaging is recommended in these patients only with clear dynamics of tumor markers, i.e., with a Ctn and/or CEA increase of >20% (1,2,20,24).
Frequent sources of error for fluctuating serum Ctn levels are transport times of the samples into the laboratory of different lengths and modalities of sample storage i.e., the temperature (3,4,12).
Patients with persistent MTC should be presented at a center with specific MTC expertise. The procedure is discussed with the institution. This is true against the background that even an MTC with distant metastases can go indolent for many years, which is why treatment recommendations always (I) the tumor biology, (II) the tumor burden, (III) the quality of life, (IV) the comorbidities and (V) the individual treatment request of the patient to take into account.
As a treatment indication for persistent MTC currently apply: (I) existing or imminent local symptoms (tracheal infiltration, esophageal/tracheal compression); (II) organ-specific complications due to distant metastases (liver, lung, bone, CNS), clinical symptoms at high tumor burden and/or hormone excess; (III) tumor progression according to the response evaluation criteria in solid tumors (RECIST); (IV) a therapy request in sufficient general condition.
A sole increase in tumor markers without morphological progression in the absence of surgical treatment indications is not an indication for therapy.
Tyrosine kinase inhibitors (TKIs)
TKIs are the drugs of first choice in the systemic therapy of MTC. Classical chemotherapy is no longer recommended (2,20). On the basis of phase III studies, TKI vandetanib (since 2012) and cabozantinib (since August 2014) have been approved. Both TKIs antagonize RET signaling in the MTC and have an antiangiogenic effect on the inhibition of the vascular endothelial growth factor (VEGF) receptor. In the Zactima Efficacy in Thyroid Cancer Assessment (ZETA) study, a total of 331 patients with locally advanced and/or metastatic MTC received vandetanib (300 mg/day) or placebo (20). The calculated median progression-free survival (PFS) was 30.5 months in the vandetanib group and 19.3 months in the placebo arm. In the Efficacy of XL184 (Cabozantinib) in Advanced Medullary Thyroid Cancer (EXAM) study, a total of 330 patients were randomized to receive either cabozantinib (140 mg/day) or placebo (21-24). In this study, only patients with a radiologic progression to RECIST were enrolled, a crossover to the verumarm was not possible. The median PFS was 11.2 months for cabozantinib and 4.0 months for the placebo group. Due to differences in patient populations and inclusion criteria, the data are not comparable in terms of potential differences in therapeutic efficacy between the two TKIs. Response to therapy is reviewed clinically and according to imaging criteria (RECIST). The tumor markers Ctn and CEA are not suitable for monitoring response to therapy, as secretion is inhibited by RET antagonization but does not necessarily have to be associated with proliferation inhibition. Both approved TKIs have general side effects, including: a. Fatigue, diarrhea, acne, rashes, photosensitivity, hypertension and altered metabolism by co-medication. In addition, there are also specific serious side effects that must be observed in the aftercare necessarily: (I) vandetanib. Long QT syndrome (ECG controls, patient card, cave: co-medication influencing QT time); (II) cabozantinib. Gastrointestinal perforation fistulization in intra-abdominal tumor manifestation intrathoracic fistula formation (except after radiation).
In addition, in almost all TKIs the levothyroxine substitution dosage has to be adjusted. Due to altered absorption and metabolism, an increase in dose is required.
According to the current state of knowledge, an essential result of TKI therapy is the disease stabilization of the MTC, which extends over a median of months. The regulatory text states that treatment should be given as long as the patient benefits clinically and toxicity is acceptable. A precise definition of this “benefit” is the subject of intense discussions. In the case of a clear progression to RECIST, a change of the TKI is currently most recommended. Results on other TKIs from phase I and phase II studies are now available at the MTC. The therapy concept is always palliative.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2018.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elisei R, Alevizaki M, Conte-Devolx B, et al. European Thyroid Association guidelines for genetic testing and its clinical consequences in medullary thyroid cancer. Eur Thyroid J 2013;1:216-31. [Crossref] [PubMed]
- Schlumberger M, Bastholt L, Dralle H, et al. 2012 European Thyroid Association guidelines for metastatic medullary thyroid cancer. Eur Thyroid J 2012;1:5-14. [Crossref] [PubMed]
- Dralle H, Musholt TJ, Schabram J, et al. German Association of Endocrine Surgeons practice guideline for the surgical management of malignant thyroid tumors. Langenbecks Arch Surg 2013;398:347-75. [Crossref] [PubMed]
- Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015;25:567-610. [Crossref] [PubMed]
- Schmid KW. Molecular pathology of thyroid tumors. Pathologe 2010;31:229-33. [Crossref] [PubMed]
- Delorme S, Raue F. Medullary Thyroid Carcinoma: Imaging. Recent Results Cancer Res 2015;204:91-116. [Crossref] [PubMed]
- Trimboli P, Giovanella L. Serum calcitonin negative medullary thyroid carcinoma: a systematic review of the literature. Clin Chem Lab Med 2015;53:1507-14. [Crossref] [PubMed]
- Sheu SY, Schmid KW. Multiple endocrine neoplasia type 2. Pathologe 2010;31:449-54. [Crossref] [PubMed]
- Frank-Raue K, Machens A, Leidig-Bruckner G, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid 2013;23:294-300. [Crossref] [PubMed]
- Ciampi R, Mian C, Fugazzola L, et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid 2013;23:50-7. [Crossref] [PubMed]
- Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 2008;93:682-7. [Crossref] [PubMed]
- Azar FK, Lee SL, Rosen JE. Medullary thyroid cancer: an update for surgeons. Am Surg 2015;81:1-8. [PubMed]
- Erovic BM, Kim D, Cassol C, et al. Prognostic and predictive markers in medullary thyroid carcinoma. Endocr Pathol 2012;23:232-42. [Crossref] [PubMed]
- Raue F. German medullary thyroid carcinoma/multiple endocrine neoplasia registry. German MTC/MEN Study Group. Medullary Thyroid Carcinoma/Multiple Endocrine Neoplasia Type 2. Langenbecks Arch Surg 1998;383:334-6. [Crossref] [PubMed]
- Führer D, Bockisch A, Schmid KW. Euthyroid goiter with and without nodules – diagnosis and treatment. Dtsch Arztebl Int 2012;109:506-15; quiz 516. [PubMed]
- Kratzsch J, Petzold A, Raue F, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clin Chem 2011;57:467-74. [Crossref] [PubMed]
- Mian C, Perrino M, Colombo C, et al. Refining calcium test for the diagnosis of medullary thyroid cancer: cutoffs, procedures, and safety. J Clin Endocrinol Metab 2014;99:1656-64. [Crossref] [PubMed]
- Machens A, Lorenz K, Dralle H. Utility of serum procalcitonin for screening and risk stratification of medullary thyroid cancer. J Clin Endocrinol Metab 2014;99:2986-94. [Crossref] [PubMed]
- Machens A, Dralle H. Biomarker-based risk stratification for previously untreated medullary thyroid cancer. J Clin Endocrinol Metab 2010;95:2655-63. [Crossref] [PubMed]
- Raue F, Frank-Raue K. Long-Term Follow-up in Medullary Thyroid Carcinoma. Recent Results Cancer Res 2015;204:207-25. [Crossref] [PubMed]
- Raue F, Frank-Raue K. Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. Recent Results Cancer Res 2015;204:61-90. [Crossref] [PubMed]
- Mitchell AL, Gandhi A, Scott-Coombes D, et al. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130:S150-60. [Crossref] [PubMed]
- Frank-Raue K, Raue F. Hereditary Medullary Thyroid Cancer Genotype-Phenotype Correlation. Recent Results Cancer Res 2015;204:139-56. [Crossref] [PubMed]
- Jin LX, Moley JF. Surgery for lymph node metastases of medullary thyroid carcinoma: A review. Cancer 2016;122:358-66. [Crossref] [PubMed]
- Skinner MA, DeBenedetti MK, Moley JF, et al. Medullary thyroid carcinoma in children with multiple endocrine neoplasia types 2A and 2B. J Pediatr Surg 1996;31:177-81; discussion 181-2. [Crossref] [PubMed]
- Marzano LA, Porcelli A, Biondi B, et al. Surgical management and follow-up of medullary thyroid carcinoma. J Surg Oncol 1995;59:162-8. [Crossref] [PubMed]
- Verbeek HH, Meijer JA, Zandee WT, et al. Fewer cancer reoperations for medullary thyroid cancer. Ann Surg Oncol 2015;22:1207-13. [Crossref] [PubMed]
- Machens A, Lorenz K, Dralle H. Individualization of lymph node dis- section in RET (rearranged during transfection) carriers at risk for medullary thyroid cancer: value of pretherapeutic calcitonin levels. Ann Surg 2009;250:305-10. [Crossref] [PubMed]
- Machens A, Dralle H. Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg 2013;257:751-7. [Crossref] [PubMed]
- Laure Giraudet A, Al Ghulzan A, Aupérin A, et al. Progression of medullary thyroid carcinoma: assessment with calcitonin and carcinoembryonic antigen doubling times. Eur J Endocrinol 2008;158:239-46. [Crossref] [PubMed]
- American Thyroid Association Guidelines Task Force. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 2009;19:565-612. [Crossref] [PubMed]
- Wells SA Jr, Robinson BG, Gagel RF, et al. () Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134-41. [Crossref] [PubMed]
- Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid can- cer. J Clin Oncol 2013;31:3639-46. [Crossref] [PubMed]
- Tiedje V, Ting S, Dralle H, et al. Medullary thyroid carcinoma. Internist (Berl) 2015;56:1019-31. [Crossref] [PubMed]
- Nixon IJ, Wang LY, Migliacci JC, et al. An International Multi-Institutional Validation of Age 55 Years as a Cutoff for Risk Stratification in the AJCC/UICC Staging System for Well-Differentiated Thyroid Cancer. Thyroid 2016;26:373-80. [Crossref] [PubMed]
- Machens A, Dralle H. Benefit-risk balance of reoperation for persistent medullary thyroid cancer. Ann Surg 2013;257:751-7. [Crossref] [PubMed]
- Kaserer K, Scheuba C, Neuhold N, et al. Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol 2001;25:1245-51. [Crossref] [PubMed]
- Engelbach M, Görges R, Forst T, et al. Improved diagnostic methods in the follow-up of medullary thyroid carcinoma by highly specific calcitonin measurements. J Clin Endocrinol Metab 2000;85:1890-4. [PubMed]
- Rohmer V, Vidal-Trecan G, Bourdelot A, et al. Prognostic factors of disease-free survival after thyroidectomy in 170 young patients with a RET germline mutation: a multicenter study of the Groupe Francais d'Etude des Tumeurs Endocrines. J Clin Endocrinol Metab 2011;96:E509-18. [Crossref] [PubMed]
- Elisei R, Pinchera A. Advances in the follow-up of differentiated or medullary thyroid cancer. Nat Rev Endocrinol 2012;8:466-75. [Crossref] [PubMed]
Cite this article as: Makay O, Bartolo V, Cancellieri A, Catalfamo A, Famà F, Pergolizzi FP, Portinari M, Donatini G, Carcoforo P, Materazzi G, Dionigi G. Medullary thyroid cancer: strategy, pitfalls and technical aspects with emphasis on remedial surgery. Ann Thyroid 2018;3:8.