
The role of prophylactic modified neck dissection in papillary thyroid cancer
About 40 years ago, there was a substantial difference between Western countries and Japan in how papillary thyroid cancer (PTC) was managed (Figure 1). Most surgeons in Western countries totally eradicated the thyroid tissue using either surgery or radioiodine, but in Japan hemithyroidectomy or subtotal thyroidectomy were considerably more common. Radioiodine is strictly regulated by law in Japan. Moreover, there is a well-developed network between the thyroid gland and neck lymph nodes (LNs). For decades, therefore, most of Japan’s thyroid surgeons used either therapeutic or prophylactic central LN dissection (p-CND) and prophylactic modified lateral neck LN dissection (p-MND) to treat PTC (1). Western thyroid surgeons, however, used only therapeutic central or lateral LN dissection.
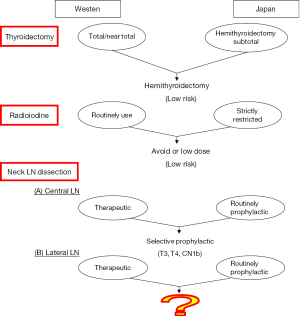
Although most thyroid cancer progresses relatively slowly, the risks of structural recurrence and mortality are classified as high, low, and intermediate (2). After recent 40 years of investigation and frequent cross talk between countries, some agreements have been reached (Figure 1) by most of the world’s thyroid surgeons: (I) a hemithyroidectomy can be used in low-risk thyroid cancer; (II) radioiodine can be avoided, or low-dose (≤30 mci) radioiodine might be adequate in low-risk thyroid cancer; and (III) p-CND for selected cases [T3, T4, or cN1b—using AJCC (8th edition) and ATA (2015 edition) criteria] reduced local recurrence (2,3).
What is the role of LN metastases in PTC? Is it an indicator or governor for survival or structural (local or distant) recurrence (4)? Many studies have suggested that lateral LN metastasis is prognostically significant for survival and local recurrence, especially in the elderly (5-12). The important factors include the number (>5) of metastatic LNs, the size of the metastatic LNs (largest metastasis >3 cm), and extranodal invasion. Most of the data rely on therapeutic lateral LN dissection (cN1b were preoperatively detected using high-resolution ultrasound or palpation). In the current follow-up strategy after initial treatment of PTC, recurrent LN metastases are easily detected using ultrasound before advanced node metastasis (involvement of great vessel or nerve) occurs. Because major postoperative complications rarely occur during the 1st MND done by experienced surgeons, p-MND has not been as emphasized as has p-CND because the rates of recurrent nerve and parathyroid injuries are much higher in reoperations for central LN recurrence. There are no studies published before 2007 that discuss the role of p-MND in PTC; the 2007 study by Ito et al. (13) found that for cN0 and cN1a PTCs, being male, being ≥55 years old, having a tumor >3 cm, and having a massive extrathyroidal extension are all independent risk factors of PTC recurrence in a LN despite having undergone a prophylactic lateral node dissection (13,14). A more recent study from the same group—Kuma thyroid center, Japan tried to verify previous conclusion and give p-MND a suitable role in PTC treatment by using a more sophisticated preoperative and postoperative evaluation system (15). A metastasis-free LN is defined as one that has negative results on (I) high-resolution ultrasound, or negative results on (II) fine needle aspiration cytology and thyroglobulin measurement of aspiration fluid for any suspicious lesion or both (I) and (II). The study (15) reported that p-MND significantly increased LN recurrence-free survival for selected cN0 and for cN1a 3.1 to 4.0-cm PTCs with significant extrathyroidal extension-positive T3b and T4 tumors [using AJCC (8th edition) personally confirmed with study authors]. During 2007–2012, p-MND was only selectively used for cN0 and cN1a patients with >3 cm PTC or a significant extrathyroidal extension (in 9% patients), and the local recurrence rate of all those cN0 or cN1a patients in the selective period 2007–2012 did not increase, compared with those cN0 or cN1a patients in the period of routinely p-MND during 1997–2005. The authors concluded that using p-MND for cN0 and cN1a PTCs measuring <4 cm did not increase the LN recurrence-free survival of patients, except for those with PTCs measuring 3.1 to 4.0-cm and with significant extrathyroidal extensions (T3b, T4) (15). This conclusion is somewhat similar to the recommendation of p-CND as suitable for T3a, T3b, T4, and cN1b tumors in the ATA guidelines (2015 edition). We expect that a good analysis between p-MND and T3a (>4 cm, but limited to the thyroid gland) comes soon from Kuma group.
Interestingly, the rate of micrometastasis in lateral LNs was up to 53–65% during p-MND (15), which revealed an excellent neck LN network with the thyroid gland, the characteristics of which tend to increase the metastasis of PTC (1,9,16,17). The predicted rate of LN micrometastasis using preoperative ultrasound is low. However, in addition to surgical removal, radioiodine is especially effective treatment against the micrometastasis of thyroid cancer. In this series, only 21 of 10,366 patients were treated with radioiodine (15), which revealed the natural course and outcomes of PTC using a purely surgical approach. Although the major complications (nerve and large vessel damage, Horner syndrome, and chyle leakage) are uncommon in experienced hand, patients often have neck discomfort, pain, and stiffness after p-MND (18). This study confirms that p-MND can be avoided for PTC patients with T1, T2, and cN0 and cN1a tumors (15).
In countries in which radioiodine is easily available, T3 and T4 patients will often be recommended for adjuvant radioiodine (dose ≥100 mci) therapy. How does adjuvant radioiodine affect the micrometastasis of lateral LNs for T3 and T4 PTC patients without p-MND? In order to reveal more specific contribution for surgery and radioiodine, additional further cohort studies on this topic should probably include four groups: (I) both p-MND and radioiodine; (II) only p-MND, no radioiodine; (III) no p-MND, only radioiodine(+); (IV) neither p-MND, nor radioiodine.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by Section Editor Qiang Zhang, MD (Department of Thyroid Surgery, the First Hospital of Jilin University, Changchun, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/aot.2017.11.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noguchi S, Murakami N. The value of lymph-node dissection in patients with differentiated thyroid cancer. Surg Clin North Am 1987;67:251-61. [Crossref] [PubMed]
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1-133. [Crossref] [PubMed]
- Tuttle RM, Morris LF, Haughen BR, et al. Thyroid-Differentiated and Anaplastic Carcinoma. In: AJCC Cancer Staging Manual. 8th edition. New York, Springer, 2017:873-90.
- Cady B. Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Ann Surg Oncol 2007;14:1790-800. [Crossref] [PubMed]
- Yamashita H, Noguchi S, Murakami N, et al. Extracapsular invasion of lymph node metastasis is an indicator of distant metastasis and poor prognosis in patients with thyroid papillary carcinoma. Cancer 1997;80:2268-72. [Crossref] [PubMed]
- Shaha AR. Prognostic factors in papillary thyroid carcinoma and implications of large nodal metastasis. Surgery 2004;135:237-9. [Crossref] [PubMed]
- Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004;135:139-48. [Crossref] [PubMed]
- Nixon IJ, Wang LY, Palmer FL, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery 2014;156:137-46. [Crossref] [PubMed]
- Wang TS, Dubner S, Sznyter LA, et al. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg 2004;130:110-3. [Crossref] [PubMed]
- Bardet S, Malville E, Rame JP, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol 2008;158:551-60. [Crossref] [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Ultrasonographically and anatomopathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinoma. World J Surg 2005;29:917-20. [Crossref] [PubMed]
- Sugitani I, Fujimoto Y, Yamada K, et al. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg 2008;32:2494-502. [Crossref] [PubMed]
- Ito Y, Higashiyama T, Takamura Y, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg 2007;31:2085-91. [Crossref] [PubMed]
- Ito Y, Miyauchi A. Lateral lymph node dissection guided by preoperative and intraoperative findings in differentiated thyroid carcinoma. World J Surg 2008;32:729-39. [Crossref] [PubMed]
- Ito Y, Miyauchi A, Kudo T, et al. The Effectiveness of Prophylactic Modified Neck Dissection for Reducing the Development of Lymph Node Recurrence of Papillary Thyroid Carcinoma. World J Surg 2017;41:2283-9. [Crossref] [PubMed]
- Sachdeva HS, Chowdhary GC, Bose SM, et al. Thyroid lymphography. Arch Surg 1974;109:385-7. [Crossref] [PubMed]
- Hao RT, Chen J, Zhao LH, et al. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol 2012;38:718-24. [Crossref] [PubMed]
- Takamura Y, Miyauchi A, Tomoda C, et al. Stretching exercises to reduce symptoms of postoperative neck discomfort after thyroid surgery: prospective randomized study. World J Surg 2005;29:775-9. [Crossref] [PubMed]
Cite this article as: Huang SM, Lee CH. The role of prophylactic modified neck dissection in papillary thyroid cancer. Ann Thyroid 2017;2:13.


